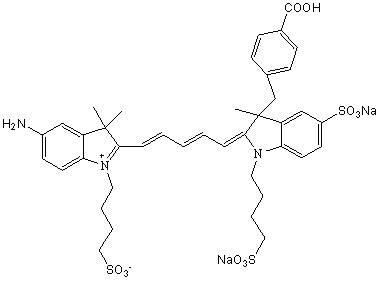
Near-infrared fluorescent probe with maximum Stoke displacement
A fluorescent probe and near-infrared technology, applied in the field of near-infrared fluorescent probes, can solve problems such as poor stability, large molecular weight of phycobiliprotein, and affecting use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Extraction of Phycocyanin
[0070] First, weigh 1 g of Porphyra zebra, add an appropriate amount of phosphate buffer, and mash the tissue. The mashed liquid is refrigerated and centrifuged at 10,000 g for 20 minutes, and the supernatant is taken. The supernatant was precipitated with 25% and 50% saturated ammonium sulfate segmented precipitation method, and the final precipitate was still dissolved in phosphate buffer solution, refrigerated and centrifuged at 15000g for 30min, the precipitate was taken out, and impurity ions were removed after dialyzing with distilled water for a period of time to obtain a crude extract. of phycocyanin. The obtained phycocyanin was cracked with 10 mol / L concentrated hydrochloric acid at 80° C. for 30 minutes, and then extracted with chloroform. The extracted solutions were combined and rotatively evaporated to remove the chloroform to obtain phycocyanin.
Embodiment 2
[0071] Example 2 Activation of Phycocyanin
[0072] 2mmol phycocyanin, 20mmol N-hydroxysuccinimide and 40mmol dicyclohexylcarbodiimide (DCC) were added to 5ml anhydrous DMF, and stirred at room temperature for 36 hours in the dark. After the reaction solution was filtered, the filtrate was added to diethyl ether to precipitate the product. Then the product was centrifuged in a centrifuge to remove the supernatant, and the solid in the lower layer was washed three times with ether, and then centrifuged in a centrifuge to remove the supernatant to obtain a crude solid. The crude product was purified by normal-phase silica gel column chromatography to obtain activated phycocyanin, which was stored in a refrigerator protected from light.
Embodiment 3
[0073] The synthesis of embodiment 3 NIR-1
[0074] Synthesis of Intermediate II
[0075] Add 10mmol of 3-(4-benzoyl)-2,3-dimethyl-3H-indole-5-sodium sulfonate (intermediate I) and 13mmol of propane sultone into a dry and clean three-necked flask , using o-dichlorobenzene as a solvent, magnetically heated and stirred to reflux under light-shielding conditions, stopped heating after 18 hours of reflux reaction, and washed the product with ether three times after the solvent was removed to obtain a reddish-brown solid product 3-(4-benzoic acid base)-2,3-dimethyl-1-(3-sulfonic acid propyl)-3H-indole-5-sodium sulfonate (intermediate II).
[0076] Synthesis of Intermediate IV
[0077] Add 10mmol of 2,3,3-trimethyl-5-nitro-3H-indole (intermediate III) and 13mmol of propane sultone into a dry and clean three-necked flask, using o-dichlorobenzene as a solvent, Under the condition of avoiding light, magnetically heat and stir to reflux, stop heating after 15 hours of reflux reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com