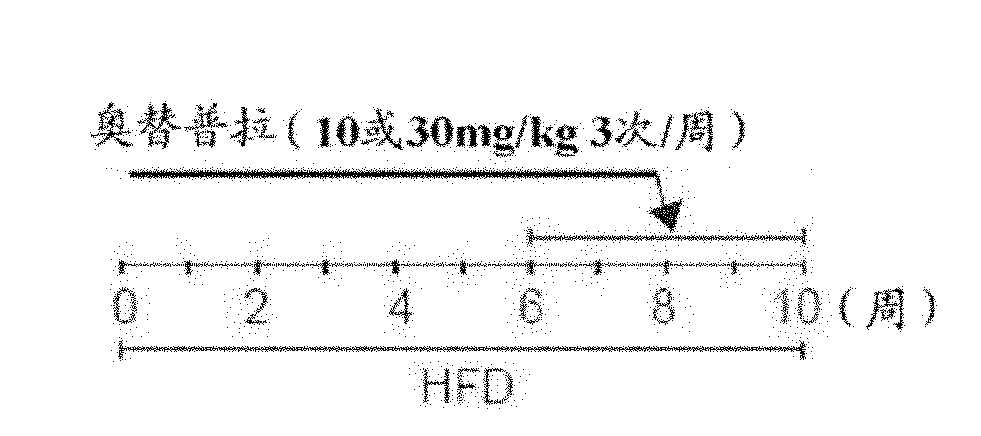Pharmaceutical composition containing 1, 2-dithiolthione derivative for preventing or treating disease caused by overexpression of LXR-alpha
A technology of overexpression and composition, which can be used in drug combinations, medical preparations containing active ingredients, metabolic diseases, etc., and can solve problems such as unproven medical or drug effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Reference Example 1: Test animals and diet
[0058] Male C57BL / 6 mice (average weight 25-30 g) were purchased from Charles River Orient (Seoul, Korea) as test animals. For at least one week before the test, the mice were acclimatized to the surrounding environment in the Animal Testing Research Center of the College of Pharmacy, Seoul National University, namely 55 ± 5% humidity, 22 ± 2° C. and controlled ventilation. At 12-hour intervals (from 7 am to 7 pm), the mice were repeatedly and alternately exposed to light and then placed in the dark. During the test, the amount of food and water consumed by the mice did not change significantly. The weight and condition of the mice were measured once a week. Two groups of mice were fed with high-fat diet (Dyets Inc., Bethlehem) and normal diet for 10 weeks. For the last four weeks in each case, oltipraz (10 or 30 mg / kg, 3 times / week) was administered to the mice ( figure 1 ). Each group consists of a total of 10 mice.
[0059...
Embodiment 5
[0065] Reference Example 5: Detection Method
[0066] The data shown in the following experimental examples are obtained by using the drug calculation program. That is, through one-way quadratic variance detection (Fisher, RA, Statistical Methods for Research Workers, Edinburgh: Oliver & Boyd, 1925) to test the significance between the experimental groups, and then through the Newman Keuls method (Norman GR et al., Biostatistics: The Bare Essentials, 2000) Determine the result ( * p** p<0.01).
experiment Embodiment 1
[0067] Experimental example 1: The therapeutic effect of oltipraz on increased expression of LXRα
[0068] The mice were fed a high-fat diet and a normal diet for 10 weeks. LXRα in the liver tissue of mice fed with a high-fat diet and administered with oltipraz (10-30 mg / kg, 3 times / week) as a 1,2-dithiothione compound for the last four weeks The expression is evaluated. mRNA was isolated from liver tissue, cDNA was synthesized by RT-PCR, and then specific primers were used (mouse LXR, 5'-TGCCATCAGCATCTTCTCTG-3' (sense) and 5'-GGCTCACCAGCTTCATTAGC-3' (antisense)) Perform real-time PCR on it. The LXRα mRNA expression level of the normal diet group (ND) was set to 1, and the relative expression level of the high-fat diet group or the high-fat diet and oltipraz administration group was measured. Result in figure 2 Shown in. Therefore, it can be seen that the expression of LXRα (which is an intracellular lipid sensor) was significantly increased due to a high-fat diet (p<0.01) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com