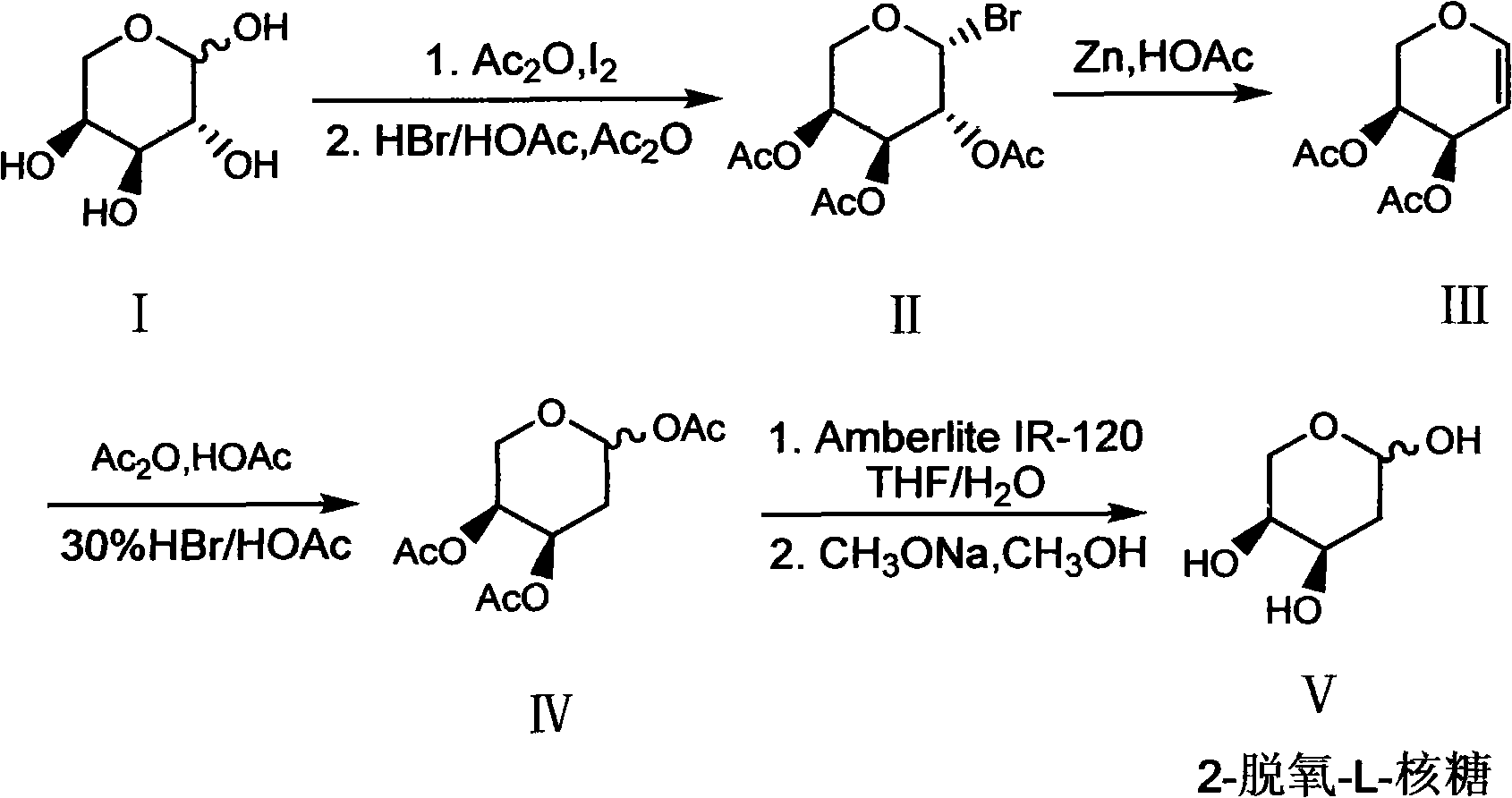
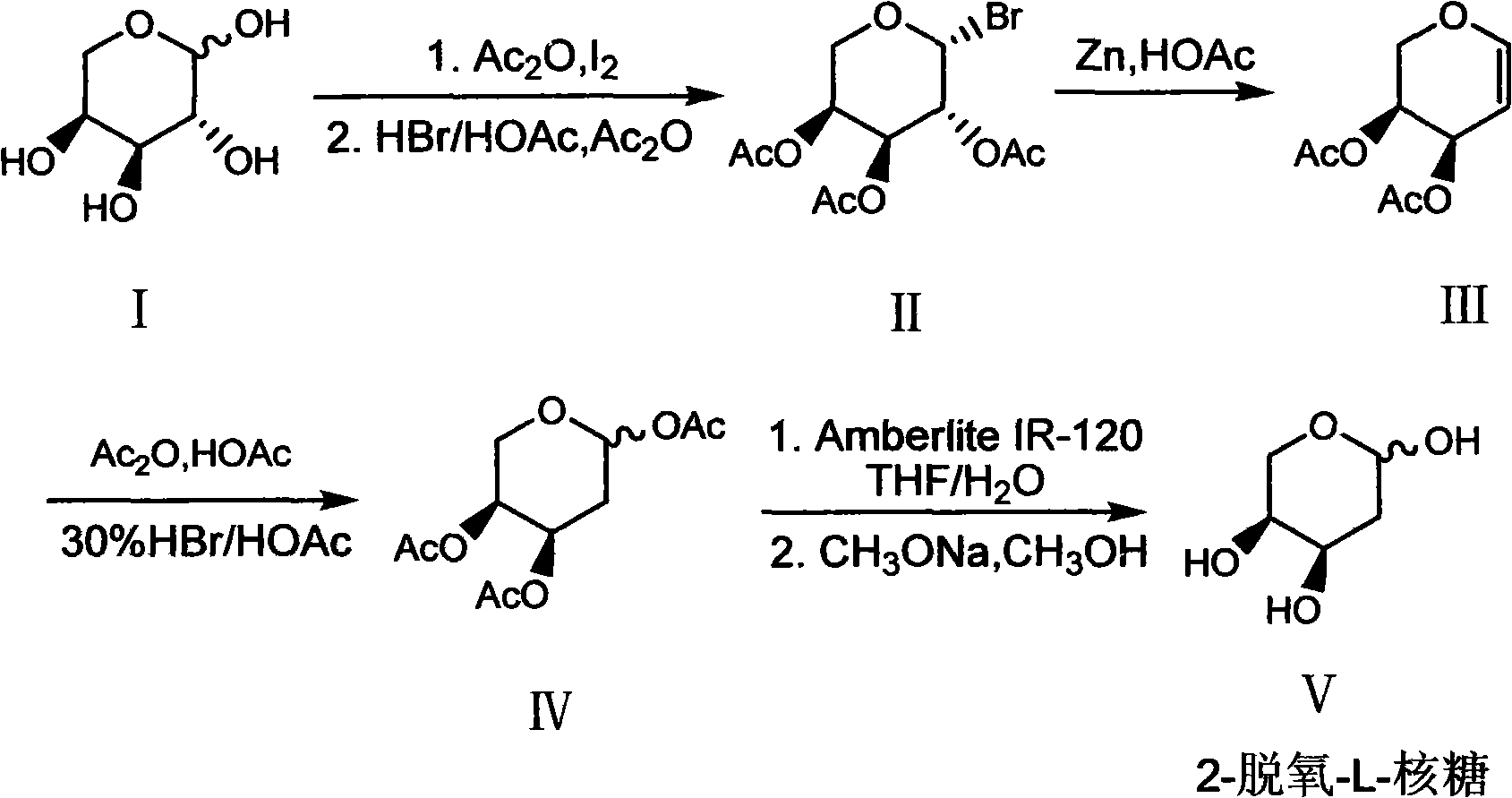
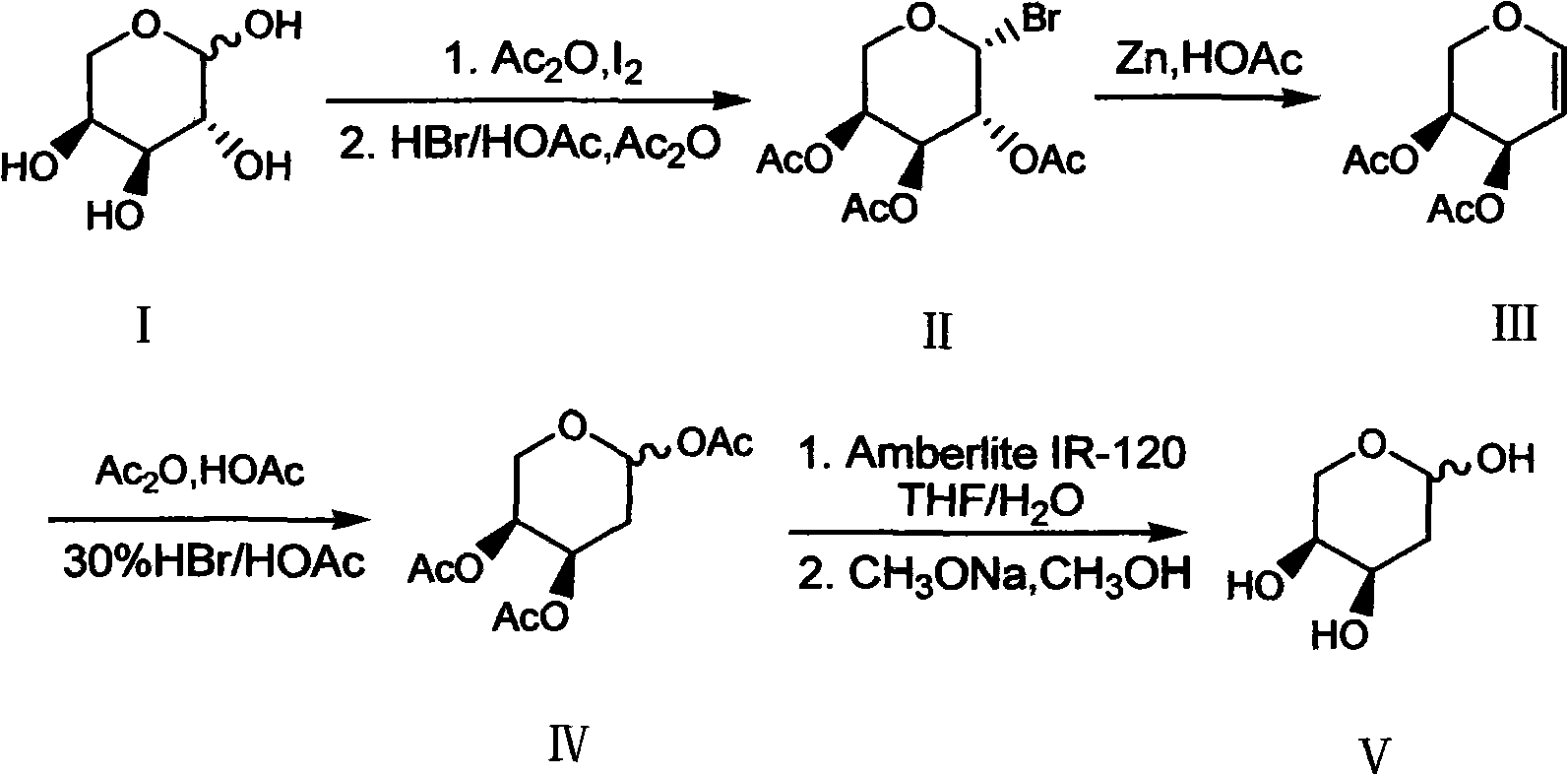
Preparation method of 2-deoxy-L-ribose
A technology for ribose and arabinose, applied in the field of preparing 2-deoxy-L-ribose, can solve the problems of complex process, large pollution and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] In order to further understand the content of the present invention, the present invention will be described in more detail with reference to preferred embodiments, and the examples described herein are provided for illustrative purposes only and do not constitute any limitation to the present invention.
[0032] (1) β-Bromo-L-triacetylarapyranose (II)
[0033] Ac 2O (300ml, 3.2mol) and L-arabinose I (100g, 0.667mol) were mixed and stirred, then added finely ground I 2 The powder (7.6 g, 0.03 mol) was stirred at room temperature for about 2 h, the arabinose was dissolved, and the system turned into a clear reddish brown. Add the above mixture to 300ml CH 2 Cl 2 Dissolve, place in an ice bath, and slowly add 30% HBr / HOAc (200ml, 1.09mol) dropwise. After the addition, the ice bath was removed and stirred at room temperature for 2 h. After the reaction was completed, add 300ml ice water to wash, and the organic layer was washed with 5% Na 2 S 2 o 3 The solution was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com