Immunochromatographic test strip for rapidly detecting malaria and preparation method thereof
A technology for immunochromatography and test strips, applied in the field of medical testing, can solve the problems of inability to display test results in multiple styles, difficult to distinguish test lines and quality control lines, and complicated preparation process of colloidal gold labels, so as to improve sensitivity and test results. accuracy, improved preventive capabilities, and significant economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
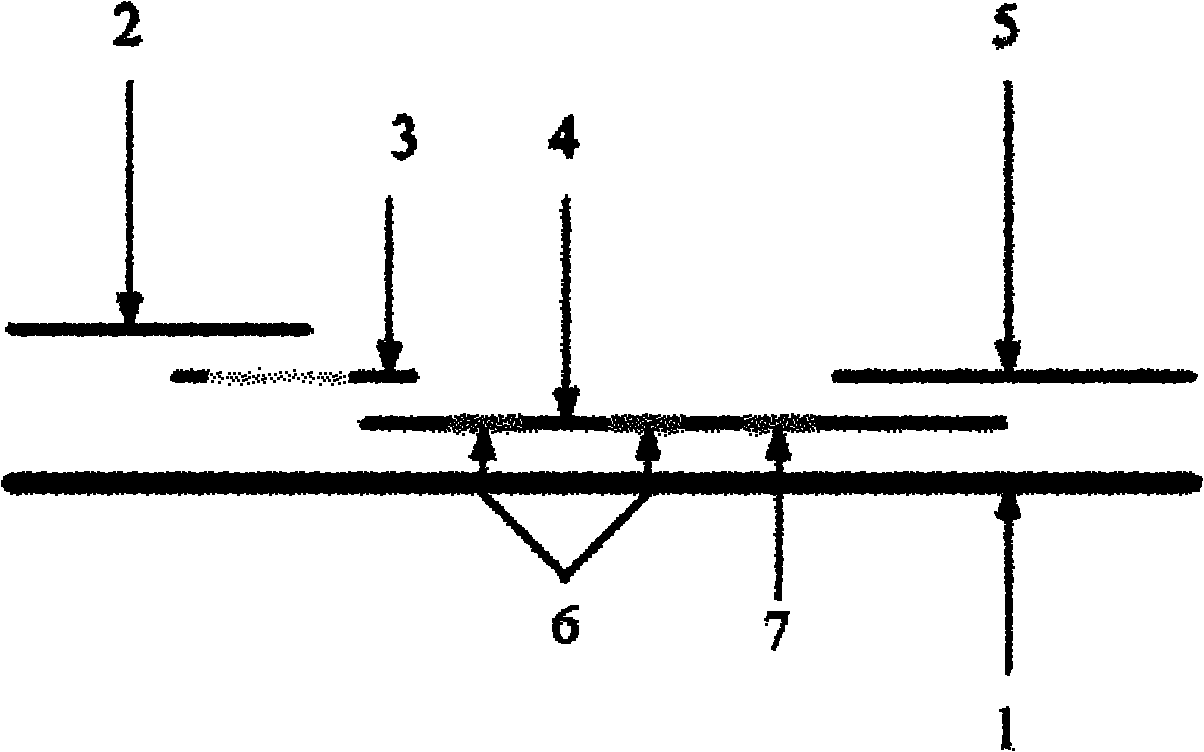
[0032] see figure 1 , is a structural schematic diagram of the malaria immunochromatographic rapid detection test strip of the present invention. A test strip for rapid detection of malaria falciparum and non-falciparum malaria provided by the present invention comprises a substrate 1 , a sample pad 2 , a marking pad 3 , a coating film 4 and absorbent paper 5 . Marker pad 3 coated with colored latex-labeled Plasmodium falciparum histidine-rich protein II (HRP-II) monoclonal antibody and non-Plasmodium falciparum lactate dehydrogenase (pLDH) monoclonal antibody, coated membrane 4 by the detection zone 6 and the control area 7, the detection area 6 includes the detection line T1 and the detection line T2, wherein T1 is coated with colored latex-labeled Plasmodium falciparum histidine-rich protein II (HRP-II) monoclonal antibody in different surface Another strain of Plasmodium falciparum histidine-rich protein II (HRP-II) monoclonal antibody; T2 is coated with a non-Plasmodium ...
Embodiment 2
[0044] The detection test strip structure in this embodiment is all identical with embodiment 1.
[0045] In this embodiment, in this embodiment, the ratio of the Plasmodium falciparum histidine-rich protein II monoclonal antibody to the colored latex particles is 1:15, and the non-Plasmodium falciparum lactate dehydrogenase monoclonal antibody The ratio of the colored latex particles to the colored latex particles is 1:15, the concentrations of the colored latex particles-labeled Plasmodium falciparum histidine-rich protein II monoclonal antibody and the non-Plasmodium falciparum lactate dehydrogenase monoclonal antibody are both 10 μg / ml, and coated The dilution parameter on the marker pad is 28cm 2 / ml. The coating concentration of the Plasmodium falciparum histidine-rich protein II monoclonal antibody in the detection zone is 1.7mg / ml; the coating concentration of the non-Plasmodium falciparum lactate dehydrogenase monoclonal antibody in the detection zone The concentrat...
Embodiment 3
[0049] The detection test strip structure in this embodiment is all identical with embodiment 1.
[0050] In this embodiment, in this embodiment, the ratio of the Plasmodium falciparum histidine-rich protein II monoclonal antibody to the colored latex particles is 1:20, and the non-Plasmodium falciparum lactate dehydrogenase monoclonal antibody The ratio of the colored latex particles to the colored latex particles is 1:20, the concentrations of the colored latex particle-labeled Plasmodium falciparum histidine-rich protein II monoclonal antibody and the non-Plasmodium falciparum lactate dehydrogenase monoclonal antibody are both 15 μg / ml, and coated The dilution parameter on the marker pad is 25cm 2 / ml. The coating concentration of the Plasmodium falciparum histidine-rich protein II monoclonal antibody in the detection area and the coating concentration of the non-Plasmodium falciparum lactate dehydrogenase monoclonal antibody in the detection area are both 2.0mg / ml; the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com