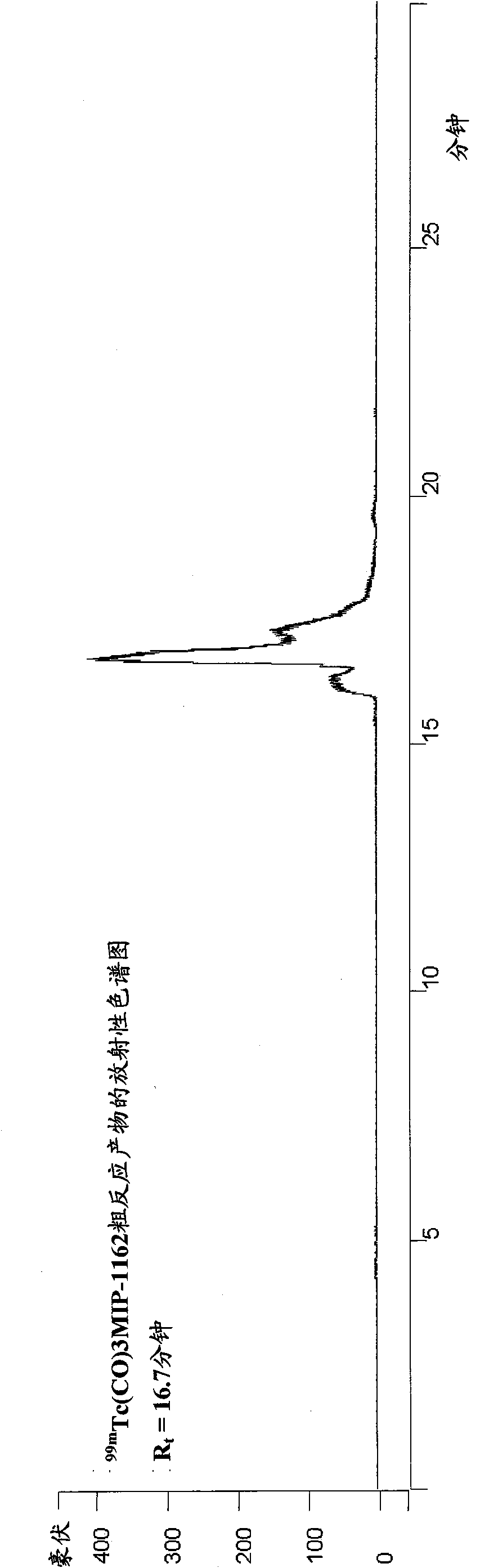
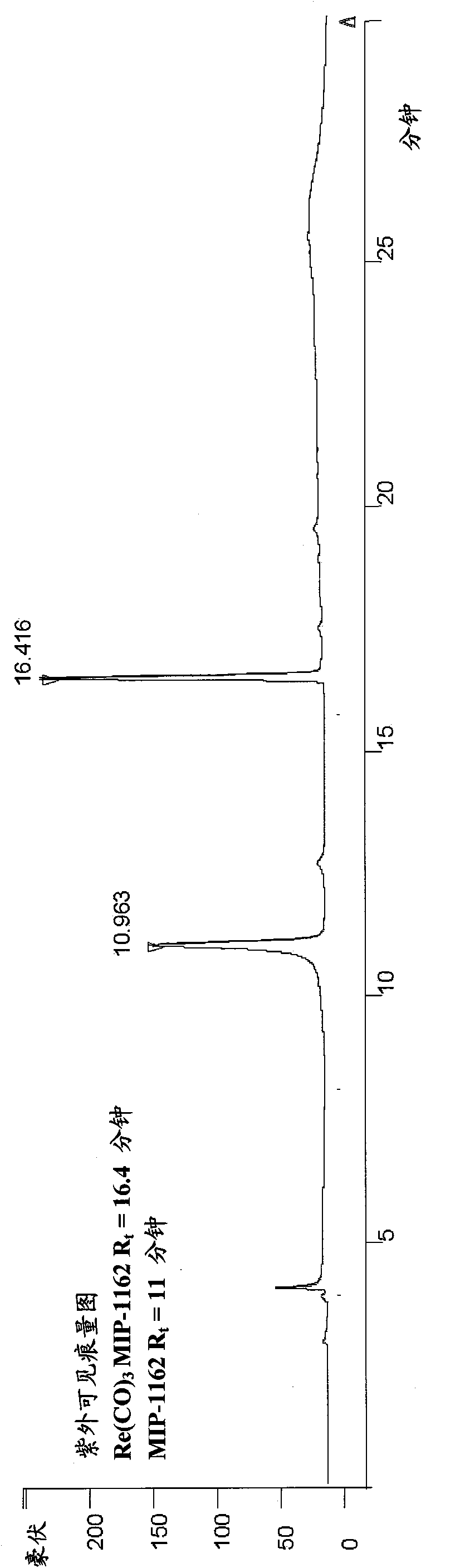
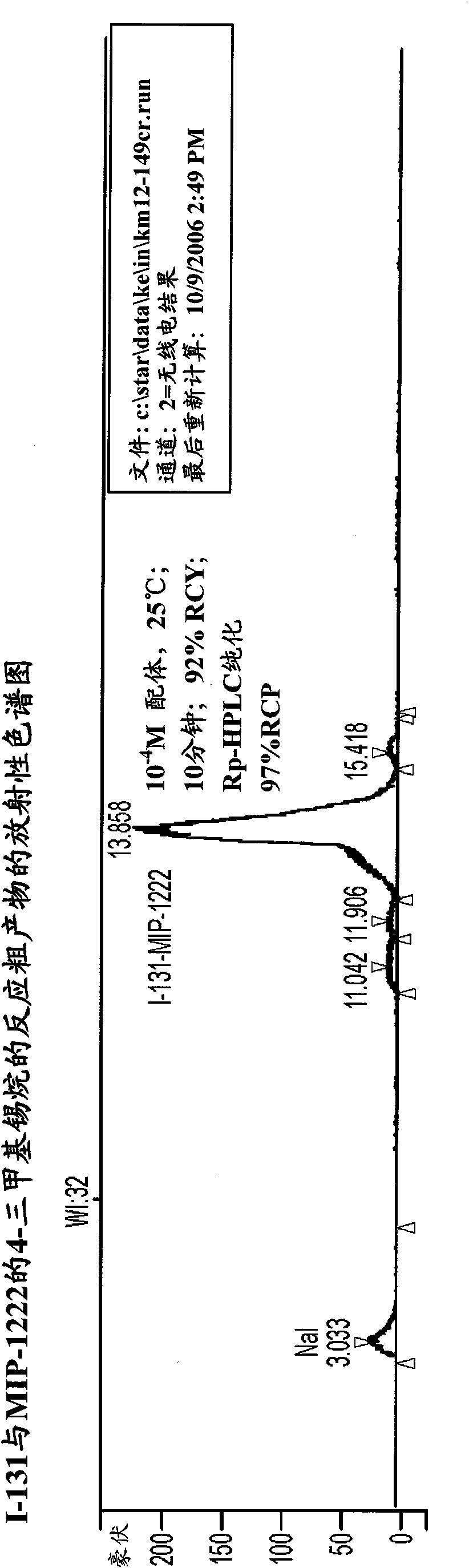
Inhibitors of carbonic anhydrase IX
A general formula, complex technology, applied in the field of radiolabeled inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0390]
[0391] 4-(bis(pyridin-2-ylmethyl)amino)benzenesulfonamide (2a)
[0392]A suspension of sulfonamide (1a, 5.0 g, 29 mmol) and pyridine-2-carbaldehyde (6 mL, 64 mmol) in EtOH (100 mL) was heated to reflux and stirred at room temperature for 30 minutes. The solvent was removed in vacuo to give a yellow solid. The resulting residue was dissolved in EtOH (100 mL), cooled to 0° C., and sodium triacetoxyborohydride (4.4 g, 120 mmol) was added portionwise. The resulting mixture was stirred at 0°C for 1 hour, then allowed to warm to room temperature for 3 hours. 2N sodium hydroxide (25 mL) was carefully added to quench the reaction. The resulting mixture was poured into saturated sodium bicarbonate (200 mL) and extracted with DCM (3×150 mL). Dry (Na2SO4) and concentrate the combined organic extracts. Flash column chromatography as a gradient elution (DCM / MeOH, 1:0 to 9:1), followed by crystallization from ethyl acetate afforded 2a as a white solid (3.41 g, 33%). 1 H NMR...
Embodiment 2
[0410] 4-Oxo-4-(4-sulfamoylphenethylamino)butanoic acid
[0411] Combine 4-(2-aminoethyl)benzenesulfonamide (1 g, 5.0 mmol) and succinic anhydride (500 mg, 5.0 mmol) in a round bottom flask containing dioxane (100 mL) and heat the slurry to reflux overnight. The white solid was filtered and washed with cold dioxane to give the desired product as a white solid (1.4 g, 4.6 mol, 92%). 1 H NMR (400MHz, DMSO-d 6 )δ8.00(s, 1H), 7.71(d, J=8.1Hz, 2H), 7.36(d, J=8.1Hz, 2H), 7.38(s, 1H), 3.26(m, 2H), 2.75( t, J=7.1Hz, 2H), 2.5(m, 2H), 2.3(t, J=7.1Hz, 2H); (M+H) + (301).
[0412]
[0413] Rhenium(I)N tricarbonyl bromide 1 -(4-(bis(pyridin-2-ylmethyl)amino)butyl)-N 4 -(4-sulfamoylphenethyl)succinamide
[0414] 4-Oxo-4-(4-sulfamoylphenethylamino)butanoic acid (210mg, 0.68mmol) and tricarbonylrhenium(I) bromide (N 1 , N 1 - bis(pyridin-2-ylmethyl)propane-1,4-diamine) (404 mg, 1.34 mmol) was dissolved in DMF (2 mL) and DIPEA (1.5 mL). The slurry was stirred at room temperature u...
Embodiment 3
[0417] tert-Butyl 6-(bis(pyridin-2-ylmethyl)amino)hexylcarbamate
[0418]Commercially available BOC-1,6-diaminohexane (5.0 g, 23.5 mmol) was added to DEC (250 mL), and 2-pyridinecarbaldehyde (4.1 mL, 51.7 mmol). The solution was stirred at room temperature for 10 minutes, then sodium triacetoxyborohydride (11.5 g, 54 mmol) was added in one portion. The solution was stirred overnight at room temperature. The bright yellow solution was evaporated to dryness, treated with 2N sodium hydroxide (150 mL) and extracted with DCM (4×150 mL). The organic extract was dried over sodium sulfate and concentrated to give a yellow oil (7.15 g, 76% yield). (M+H) + (399).
[0419]
[0420] Tricarbonylrhenium(I) bromide tert-butyl 6-(bis(pyridin-2-ylmethyl)amino)hexylcarbamate
[0421] In a pressure tube, combine tert-butyl 6-(bis(pyridin-2-ylmethyl)amino)hexylcarbamate (355 mg, 0.89 mmol) into methanol (4 mL) and add Re(CO) 3 (H 2 O) 2 Br (360mg, 0.9mmol), stirred overnight at 125°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com