Fusion protein, preparation method thereof, DNA sequence for coding protein, expression vector, host cell and protein-containing medicinal compoisition
A technology of DNA sequence and fusion protein, which is applied in the field of medicine, can solve problems such as the absence of IgG immunoglobulins, and achieve the effects of small dosage, high expression and low cost in clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1 Preparation of Exendin-4 target gene and human IgG2 Fc fragment gene linked with linking peptide
[0064] The gene of the target protein containing signal peptide, Exendin-4 and G5S connecting peptide (synthesized by Shanghai Shenggong Co., Ltd.) is obtained by the method of whole gene synthesis, its nucleotide sequence is shown in SEQ ID NO: 7, and the amino acid sequence is shown in SEQ ID NO: 8 shown. Wherein, the 5' end of the nucleotide sequence contains a human I1-2 signal peptide sequence, and the 3' end contains a G5S linking peptide sequence for linking with the human IgG2 Fc fragment. In order to facilitate insertion into the cloning vector pCR2.1 (Invitrogen), a Kpn I restriction site was designed at the 5' end, and a BamH I restriction site was designed at the 3' end.
[0065] The following primers were designed to amplify the human IgG2 Fc fragment cDNA:
[0066] 5' Primer: 5'CG G GAT CC G AGC GCA AAT GTT GTG TCGAGT GC 3’
[0067] 3' Primer: ...
Embodiment 2
[0073] Example 2 Construction of recombinant plasmids
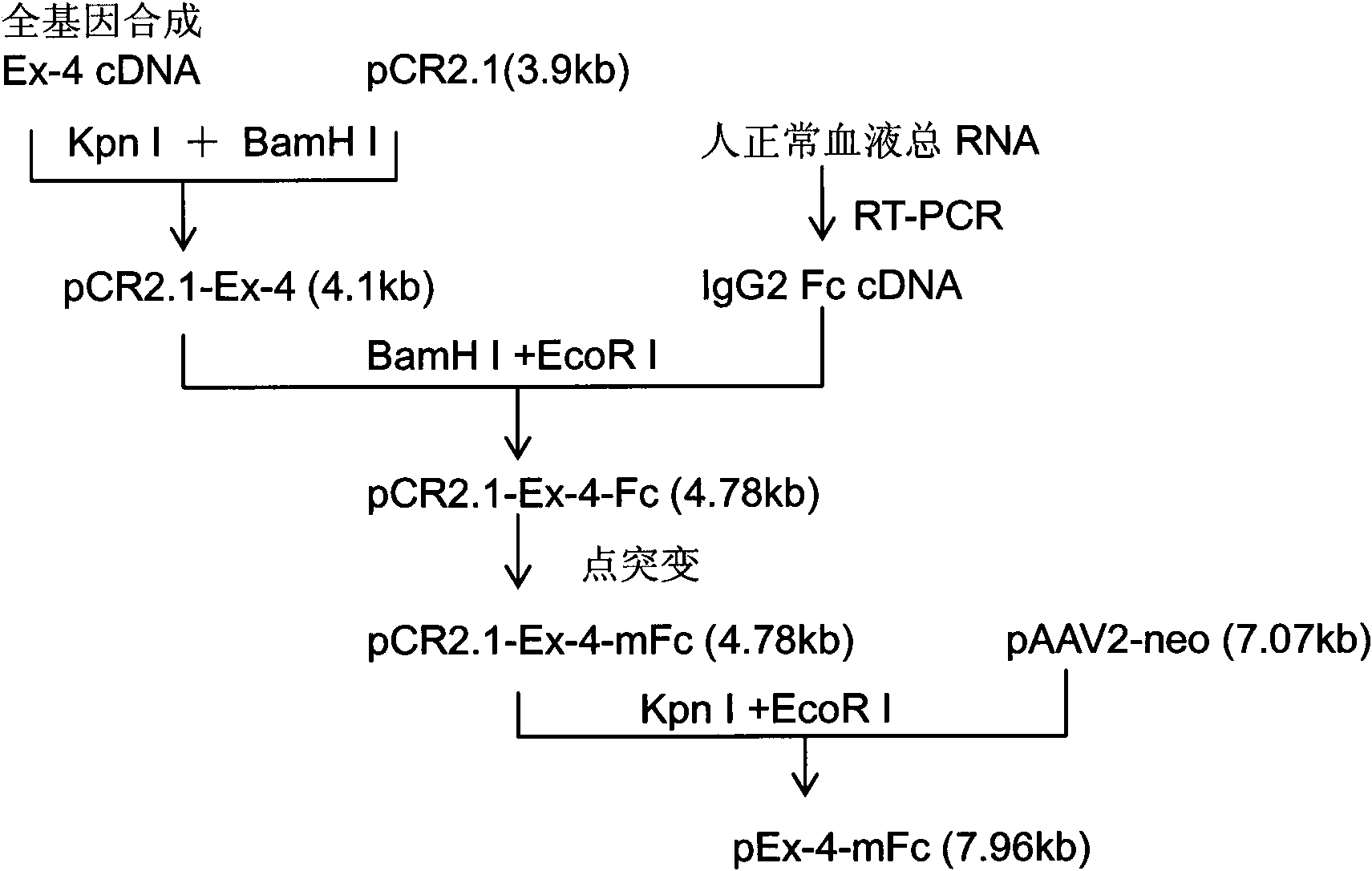
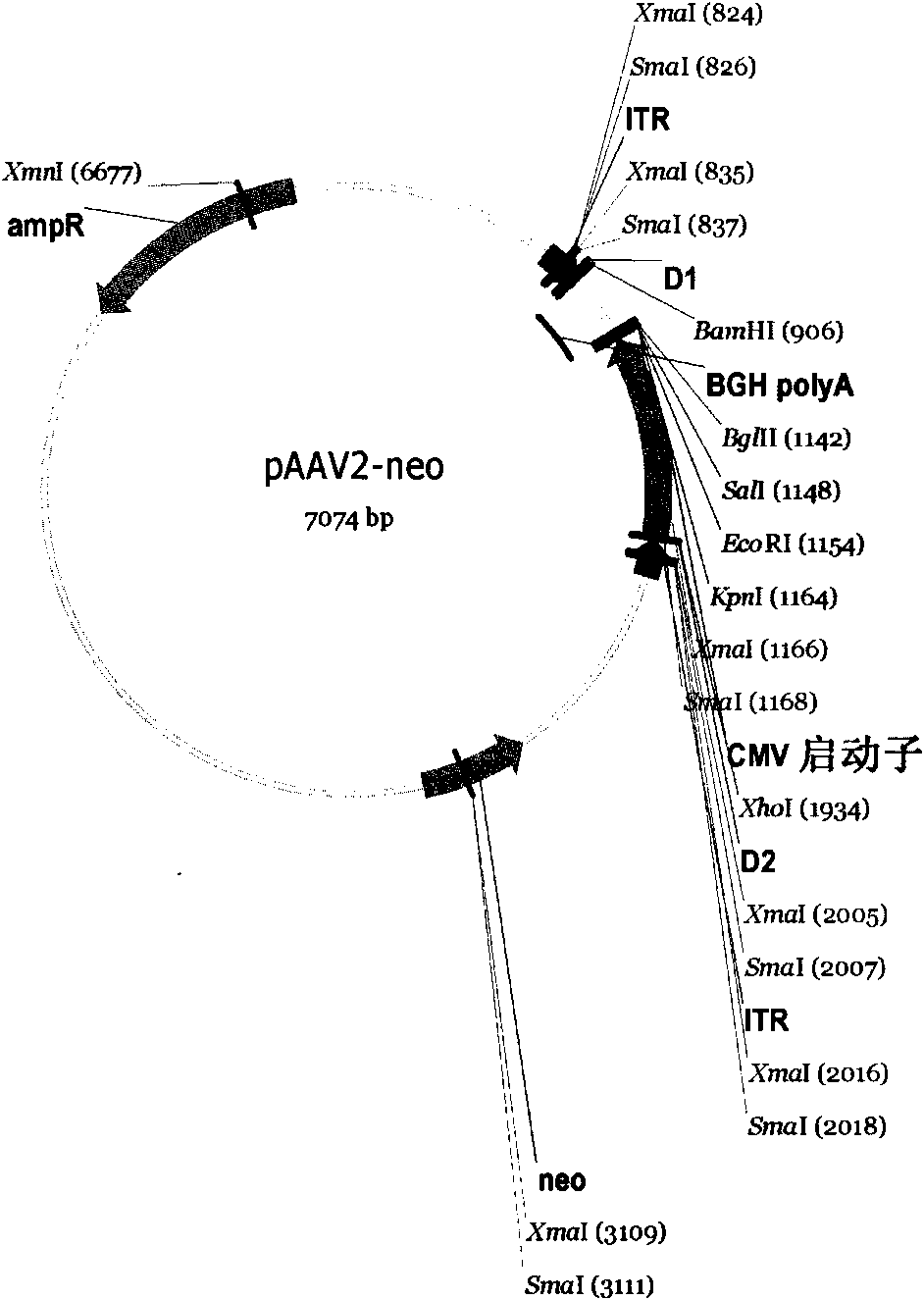
[0074] As mentioned above, a Kpn I restriction site is designed at the 5' end of the target gene, and a BamH I restriction site is designed at the 3' end, so that the target gene fragment can be directly inserted into the multiple cloning site Kpn I of the cloning vector pCR2.1 / BamH I, and then insert the human IgG2 Fc fragment into the multiple cloning site BamH I / EcoR I to obtain the Ex-4-Fc fusion fragment. A point mutation of the human IgG2 Fc fragment was carried out in the recombinant clone, and after sequencing and identification, Kpn I / EcoR I was used to insert the eukaryotic expression vector pAAV2-neo. The expression vector pAAV2-neo was transformed on the basis of pcDNA3.1 (Invitrogen), and the terminal repeat sequence of AAV2 was added to increase the stability of the integrated fragment in the genome (see image 3 ). The construction process of the recombinant plasmid is detailed in figure 1 .
[0075] 1...
Embodiment 3
[0083] Example 3 Screening and Identification of CHO Stable Expression Strains (E4F / CHO Cells)
[0084] 10 μg of the pEx-4-mFc plasmid prepared in Example 2 was used to transfect CHO-K1 cells (ATCC) with liposome Lipofectamine 2000 (Invitrogen). Two days later, the cells were subcultured at a ratio of 1:5, and 0.4 mg / ml G418 (Invitrogen) was added for selection. Colonies could be formed after 10 days. Digest 120 single clones with clear edges and good cell state and inoculate five 24-well plates at 37°C, 5% CO 2 Culture in an incubator (the first round of selection), the medium is DMEM / F12 (Invitrogen) containing 10% fetal bovine serum. Unless otherwise specified, the culture temperature and medium in the culture described below were the same as that.
[0085] The culture supernatant after three days was taken to detect the expression of the fusion protein by ELISA method, and 46 clones with positive expression were selected and inoculated in two 24-well plates respectively ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com