Preparation method of fat emulsion of cerebral protection therapeutic drug
A fat emulsion and emulsifier technology, applied in the field of preparation, can solve the problems of increased hemolysis percentage, non-advocated use, toxic and side effects, and achieve the effects of reducing toxic and side effects, saving time and cost, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
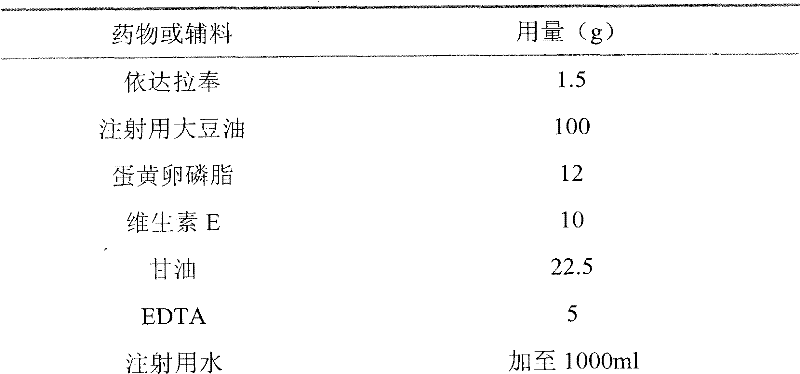
[0046] Embodiment 1 Edaravone fat emulsion injection (0.15%):
[0047]
[0048] Preparation Process:
[0049] (1) Dissolve 12g of egg yolk lecithin with 100g of soybean oil for injection in a water bath at 70°C under nitrogen protection, add 1.5g of edaravone and 10g of vitamin E, heat and stir to dissolve, and obtain an oil phase;
[0050] (2) 22.5g glycerol and 5g EDTA were dissolved in 500ml water to obtain the water phase;
[0051] (3) Slowly add the oil phase to the water phase dropwise, at the same time under the protection of nitrogen, shear (shear speed: 10000r / min) for 20min, add water for injection to 1000ml to obtain colostrum, and use sodium hydroxide or hydrochloric acid if necessary Adjust the pH value to around 7.0;
[0052] (4) Under the pressure of 800-900 bar, the colostrum was homogenized 5-8 times by a high-pressure homogenizer, filtered through a microporous membrane, filled with nitrogen, potted, and sterilized under high pressure at 115° C. to obtai...
Embodiment 2
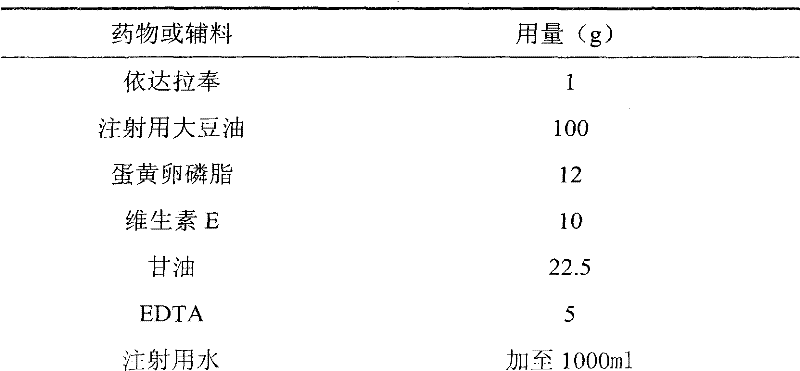
[0053] Embodiment 2 Edaravone fat emulsion injection (0.1%):
[0054]
[0055] Preparation Process:
[0056] (1) Dissolve 12g of egg yolk lecithin with 100g of soybean oil for injection in a water bath at 70°C under nitrogen protection, add 1g of edaravone and 10g of vitamin E, heat and stir to dissolve, and obtain an oil phase;
[0057] (2) 22.5g glycerol and 5g EDTA were dissolved in 500ml water to obtain the water phase;
[0058] (3) Slowly drop the oil phase into the water phase, and at the same time, shear for 20 minutes under the protection of nitrogen, replenish water for injection to 1000 ml, obtain colostrum, and adjust the pH value to about 7.0 with sodium hydroxide or hydrochloric acid;
[0059] (4) Under the pressure of 800-900 bar, the colostrum was homogenized by a high-pressure homogenizer for 5-8 times, filtered through a microporous membrane, filled with nitrogen, potted, and sterilized under high pressure at 115° C. to obtain a fat emulsion injection.
Embodiment 3
[0060] Long-chain Edaravone fat emulsion injection (0.5%) in Example 3:
[0061]
[0062] Preparation process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com