Homocysteine synthase inhibitor
A solvate and amide derivative technology, applied in the field of amide derivatives, can solve the problems of unreported SAHH inhibition, undisclosed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
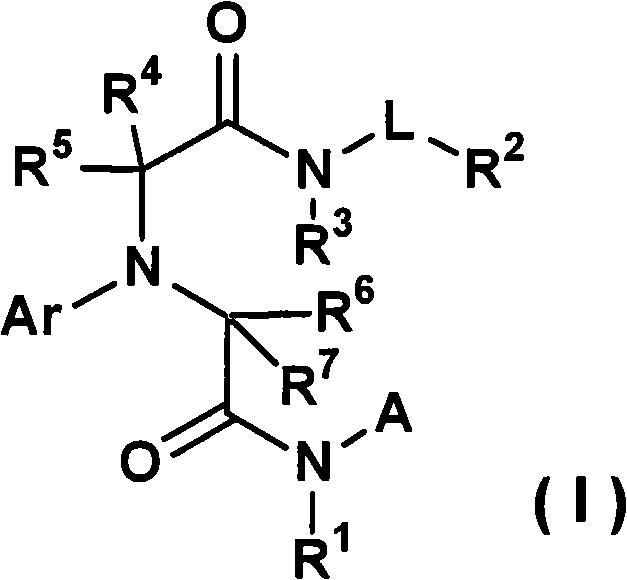
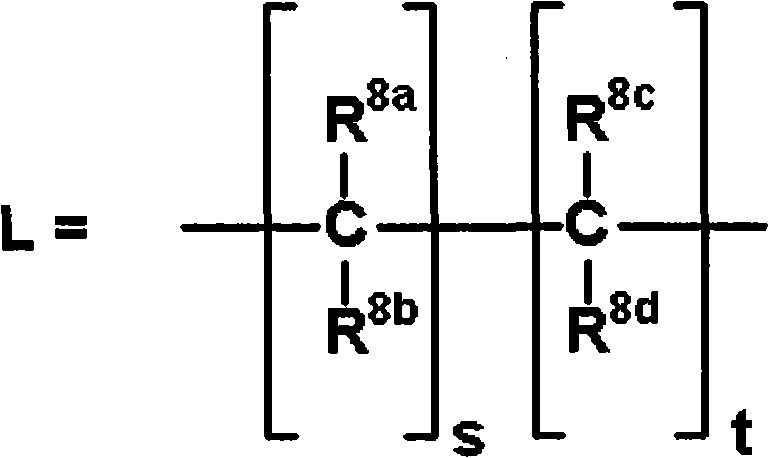
[0343] The production method of the compound of the present invention is explained below.
[0344]The compound represented by the general formula (I) or a salt thereof can be synthesized by various known synthesis methods by utilizing the characteristics based on its basic skeleton or type of substituent. Typical production methods are illustrated below, but are not limited to the methods described below. It should be noted that, depending on the type of functional group, sometimes at the stage of raw materials or intermediates, the functional group is first changed into an appropriate protecting group, that is, a group that is easily converted into the functional group is effective in preparation technology, and can be removed as needed. protecting group to give the desired compound. Examples of these functional groups include hydroxyl groups, carboxyl groups, and amino groups, and examples of these protective groups include those described in "Protective Groups in Organic S...
Embodiment
[0904] Hereinafter, although the Example of this invention demonstrates more concretely, the scope of this invention is not limited to the following Example.
[0905] "Room temperature" in the following reference examples and examples means 0 to 30°C. In addition, when a mixed solvent is used, the solvent ratio represents a volume ratio.
[0906] Mass spectra were measured by any of the following methods.
[0907]
[0908] Device: LC-2010
[0909] Column: Chromolith SpeedROD RP-18e (manufactured by Merck)
[0910] Mobile phase: A solution (0.05% trifluoroacetic acid / water), B solution (0.05% trifluoroacetic acid / acetonitrile), gradient elution from A solution: B solution = 95:5 to A solution: B solution = 0:100 4 minutes
[0911] Flow rate: 4.0ml / min
[0912] Column temperature: room temperature
[0913] MS measurement mode: ESI (Electrospray ionization) method Positive
[0914]
[0915] Device: Acquity UPLC / ZQ
[0916] Column: Acquity BEH C18 (manufactured by ...
reference example 1
[0930] (2-Aminoethyl)methylcarbamate tert-butyl hydrochloride
[0931] According to the method described in Synthetic Communications, 23(17), 2443-2449(1993), tert-butyl (2-aminoethyl)methylcarbamate was obtained from 10 g of N-methylaminoethanol. Use 4 equivalents of hydrochloric acid-di The hydrochloride salt was obtained from an alkane solution to obtain 9 g of the title compound as a colorless solid.
[0932] 1 H-NMR (300MHz, DMSO-d 6 ); δ(ppm) 1.41(s, 9H), 2.81(s, 3H), 2.89(q, J=6.1, 2H), 3.39(t, J=6.5, 2H), 8.05(brs, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com