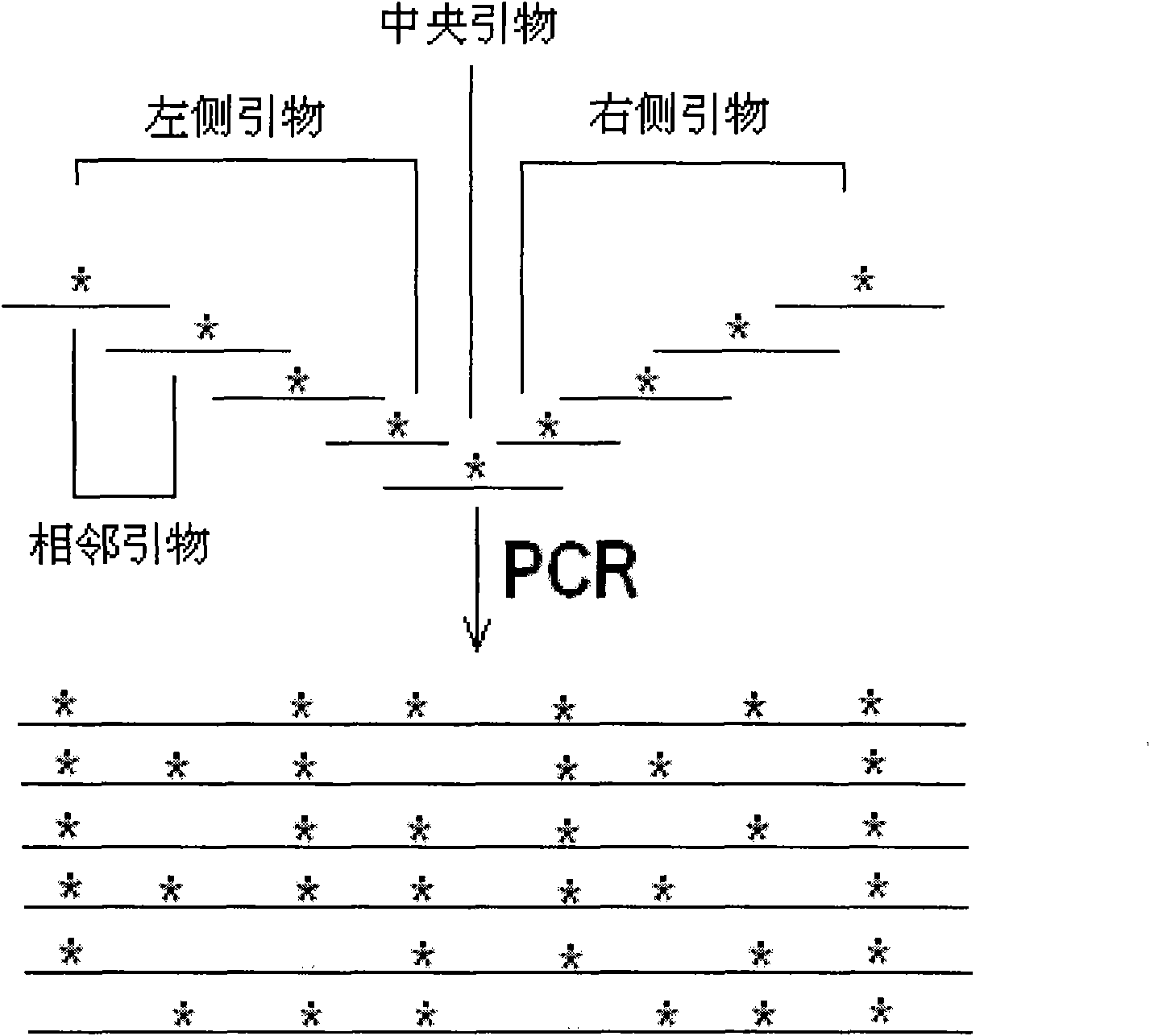
Goose-array type localized random mutation method and application thereof in monoclonal antibody molecular evolution technology
A technology of random mutation and molecular evolution, applied in the biological field, can solve the problems of long time, huge investment and high risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Example 1 Construction of a fully human ultra-large-scale antibody library
[0131] The construction of a fully human ultra-large-scale antibody library was carried out according to the methods in references 1-10. The specific operation is as follows:
[0132] 1. Blood Sample Collection and cDNA Synthesis
[0133] Collect 1ml of peripheral blood from 3000 people, mix them, and separate mononuclear cells with lymphocyte separation medium (produced by Tianjin Institute of Blood, Academy of Medical Sciences). Total mRNA was extracted from isolated human peripheral blood lymphocytes using a kit from GIBCO. The mRNA purification kit from GIBCO was used to purify, and the mRNA obtained above was used as a template to reverse transcribe the first strand of cDNA. The above steps were carried out according to the instructions provided by the manufacturer.
[0134] 2. PCR amplification
[0135] 1. Primer Design
[0136] In order to construct a fully human Fab antibody libra...
Embodiment 2
[0160] The fully human anti-human TNFa monoclonal antibody gene of embodiment 2 people's B cell origin obtains
[0161] In addition to the above-mentioned pathways, there are other sources of fully human monoclonal antibody genes for some antigens, such as human leukocytes that secrete anti-human TNFα monoclonal antibodies.
[0162] 1. Blood samples and their initial screening
[0163] Take 5 milliliters of peripheral blood from patients with active rheumatoid arthritis, separate leukocytes with lymphocyte separation medium, culture them, and identify positive clones according to the results of ELISA.
[0164] In order to obtain human B cells that secrete anti-human TNFα, a 96-well plate was routinely coated with recombinant human TNF (Shanghai Xinbainuo Bioengineering Co., Ltd.), and each well was coated with 250ng of the protein overnight. Then, it was blocked with 5% skimmed milk powder at room temperature for 2 hours, and the milk powder was prepared with pH7.2 PBS. Afte...
Embodiment 3
[0190] Example 3, panning and screening anti-human TNFα monoclonal antibody from a fully human antibody library
[0191] Based on the antibody library established in Examples 1 and 2, the operation steps of panning are as follows. During panning and other processes, the Fab form of Humira was used as a positive control.
[0192] 1. Add 1 ml of recovered antibody library strains to 14 ml of fresh LB medium, and culture in a 50 ml Erlenmeyer flask at 37°C for 16 hours.
[0193] 2. Centrifuge at high speed at 12000rpm for 10 minutes, transfer the supernatant to a sterile 50ml centrifuge tube, and save it for later use. Its titer should be above 2X10E11.
[0194] 3. Using purified recombinant human TNFa (provided by Shanghai Xinbainuo Company) as an antigen, the 25 ml cell culture flask was coated by a conventional method. Add no less than 3X10E10 phage particles to the coated cell bottle and incubate at 37°C for 1 hour.
[0195] 4. Pour off the liquid in the bottle, and wash ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com