Iloperidone crystal, and preparation method and medicinal composition thereof
A technology of iloperidone and composition, applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problem of not mentioning iloperidone crystal form, unable to obtain stable and definite crystal form, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 : Preparation method of iloperidone crystal
[0038] The present embodiment is the preparation method of iloperidone crystal, specifically comprises the following steps:
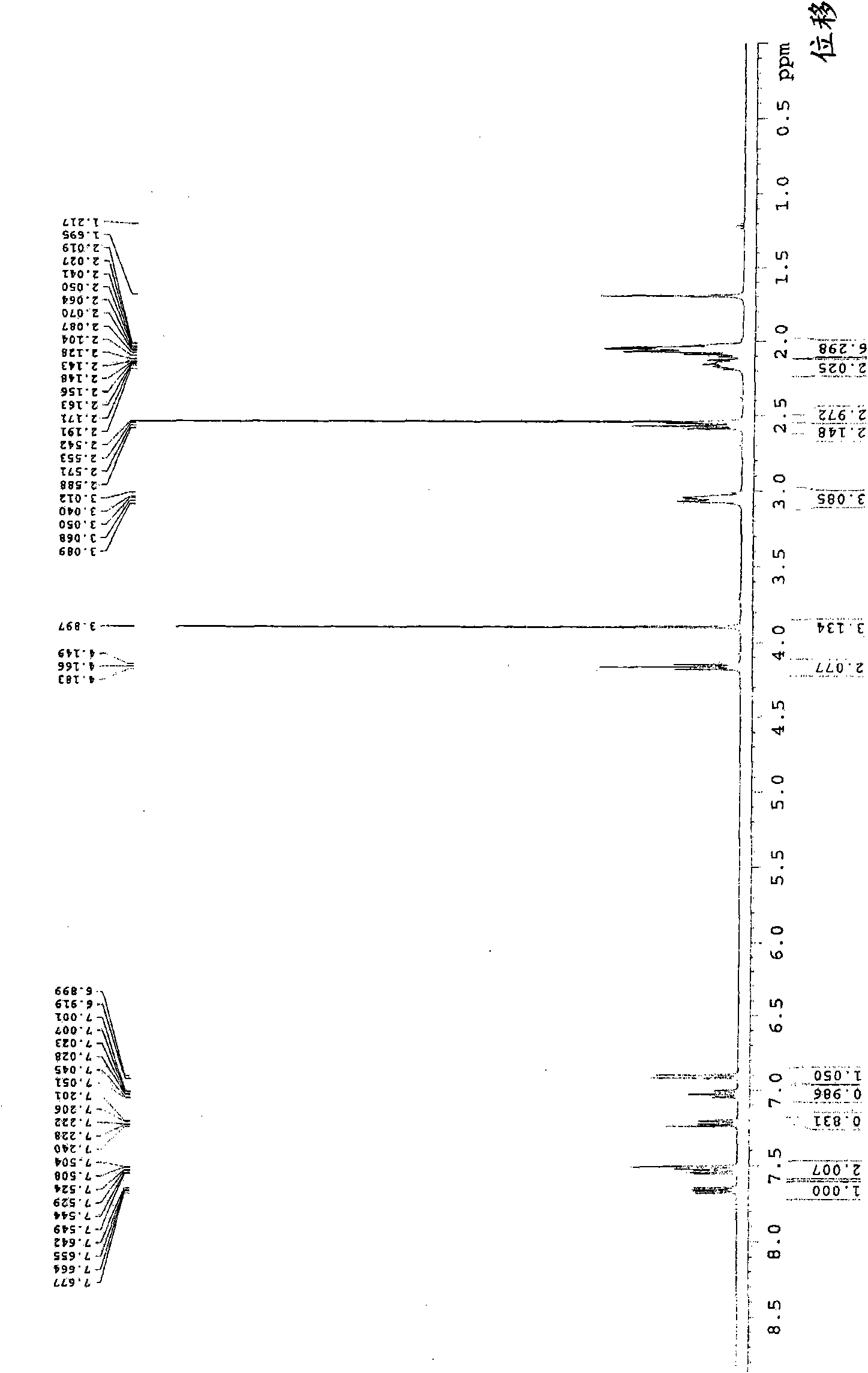
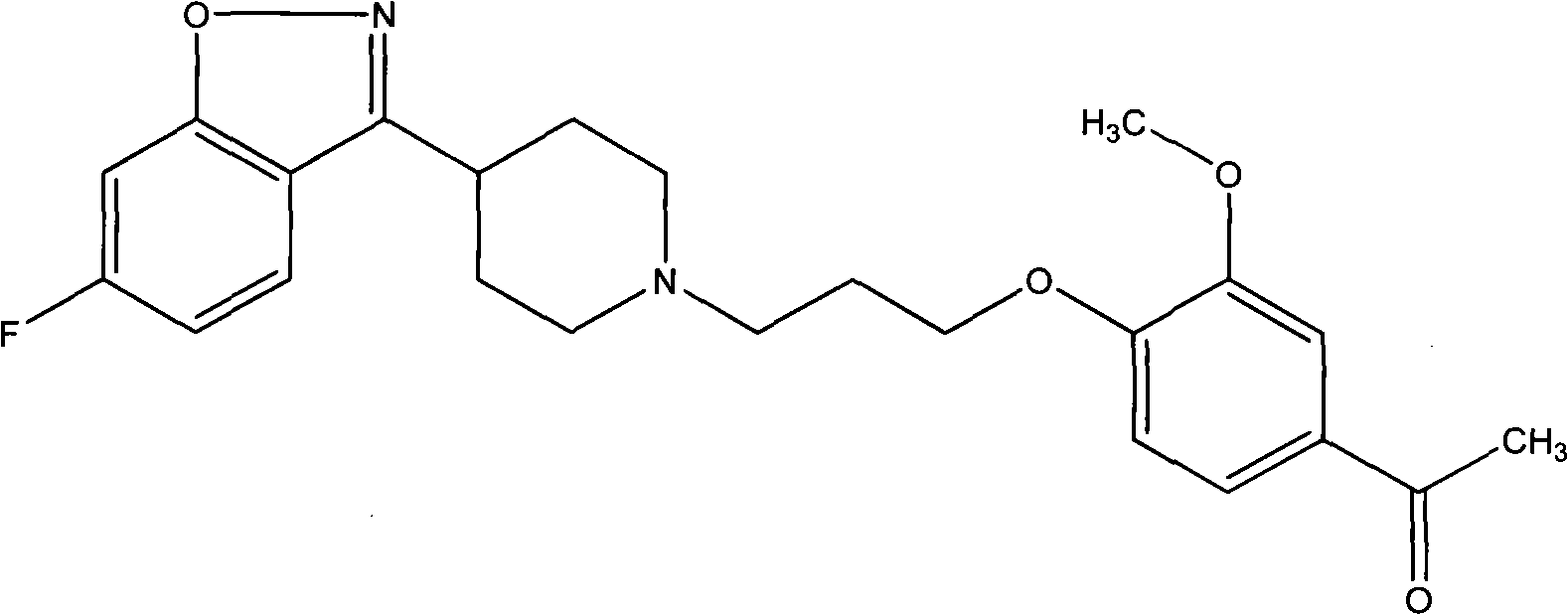
[0039] With 5g iloperidone (prepared according to the method described in EP402644A1, its nuclear magnetic spectrum is as follows figure 1 Shown) mixed with 150ml of 50% (V / V) aqueous ethanol, heated to dissolve, after the solid was completely dissolved, naturally cooled to 50°C for 30 minutes, solids were precipitated, continued to cool naturally to room temperature 25°C, and then iced The water was cooled to 10°C, left standing for 4 hours, filtered, and vacuum-dried below 60°C to obtain 4 g of off-white solid, i.e. iloperidone crystals.
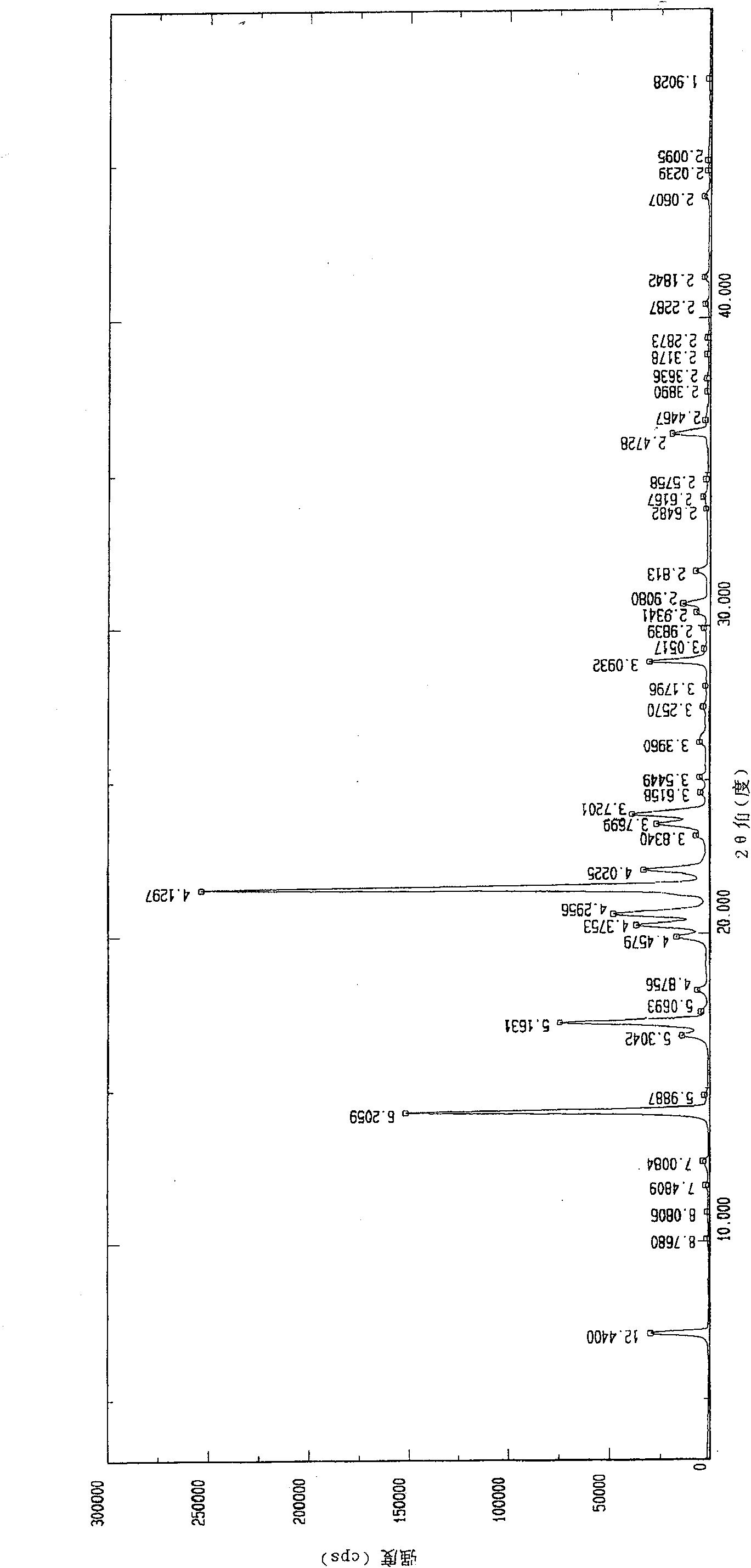
[0040] Using Japan Rigaku D / Max-2500 X-ray diffractometer (CuK α radiation) to characterize the above-prepared iloperidone crystals, the specific experimental parameters are set as follows: the 2θ angular scanning speed is 8°C / min, the scanning range...
Embodiment 2
[0043] Embodiment 2: Research on the physical properties of iloperidone crystals of the present invention
[0044] The physical properties of the iloperidone crystals of the present invention, such as solubility, hygroscopicity and stability, are studied below by comparing the iloperidone crystals of the present invention with iloperidone prepared according to the method described in EP402644A1.
[0045] 1. Solubility
[0046] According to the Chinese Pharmacopoeia 2005 edition two general example XIII method detection according to the dissolubility of iloperidone (control sample) prepared by the method described in EP402644A1 and iloperidone crystal of the present invention in 0.1M hydrochloric acid, the results are shown in Table 2 shown.
[0047] Table 2 Solubility comparison in 0.1M hydrochloric acid
[0048] sample name
0.1M hydrochloric acid
Control sample (10mg dosage)
200ml
insoluble
Iloperidone crystal (10mg dosage)...
Embodiment 3
[0062] Example 3 : Pharmaceutical composition comprising iloperidone crystals
[0063] This embodiment is a pharmaceutical composition comprising iloperidone crystals prepared in embodiment 1.
[0064] The pharmaceutical composition is prepared into tablets, and the specific prescription is as follows (1000 tablets, each containing 1 mg, 4 mg or 6 mg of active ingredient iloperidone crystal):
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com