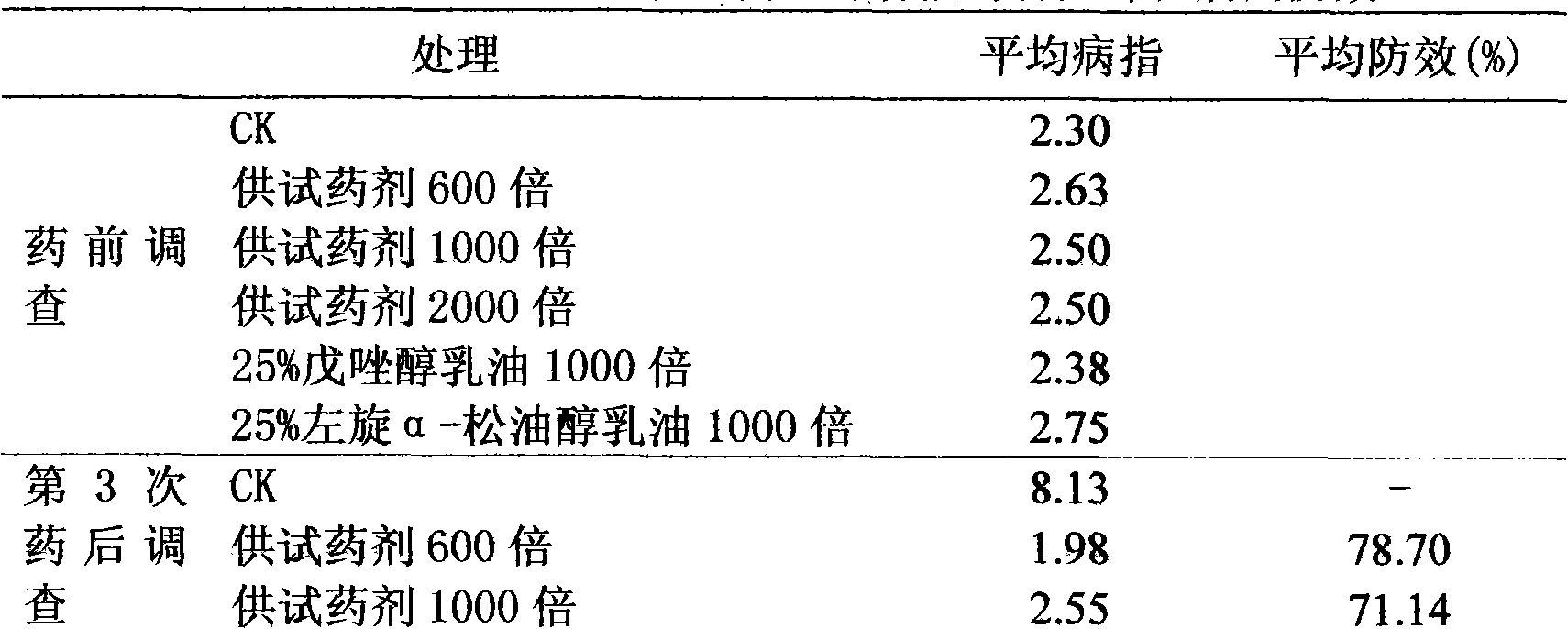
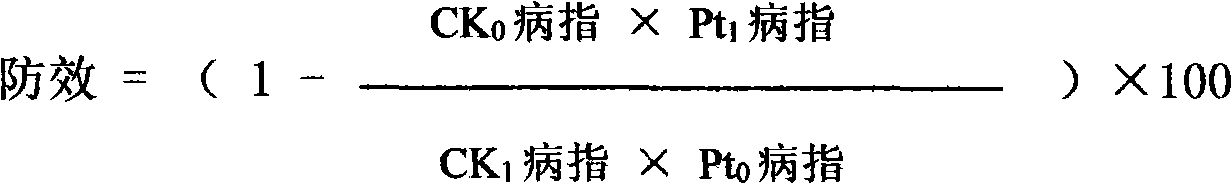Disinfectant composition comprising tebuconazole and levorotatory alpha-terpilenol and production method thereof
A fungicide composition and tebuconazole-containing technology, applied in fungicides, botany equipment and methods, biocides, etc., can solve the problems of tebuconazole research and application that have not been reported in open literature, and achieve delay drug resistance Individual production, long action time and duration, and the effect of expanding the scope of bactericidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 (12.5% Tebuconazole L-α-Terpineol EC):
[0034] (1) Raw material ratio:
[0035] By weight percentage, tebuconazole 10%, L-α-terpineol 2.5%, calcium dodecylbenzenesulfonate 4%, castor oil polyoxyethylene ether 3%, fatty alcohol polyoxyethylene ether 3%, xylene 30%, Methylated Rapeseed Oil supplemented to 100%.
[0036] (2) Preparation method:
[0037] Add tebuconazole, L-α-terpineol, calcium dodecylbenzenesulfonate, castor oil polyoxyethylene ether, fatty alcohol polyoxyethylene ether, and xylene into the methylated rapeseed oil and stir until homogeneous. Phase liquid, obtains the composition emulsifiable concentrate that active ingredient content is 12.5%.
Embodiment 2
[0038] Example 2 (25% Tebuconazole L-α-Terpineol EC):
[0039] (1) Raw material ratio:
[0040]By weight percentage, tebuconazole 20%, L-α-terpineol 5%, calcium dodecylbenzenesulfonate 4%, nonylphenol polyoxyethylene ether 3%, phenethylphenol polyoxyethylene ether 3% , supplemented with toluene to 100%.
[0041] (2) Preparation method:
[0042] Add tebuconazole, L-α-terpineol, calcium dodecylbenzenesulfonate, nonylphenol polyoxyethylene ether and phenethylphenol polyoxyethylene ether into toluene in sequence and stir until homogeneous liquid is obtained. Composition emulsifiable concentrate with an ingredient content of 25%.
Embodiment 3
[0043] Example 3 (40% Tebuconazole·L-α-Terpineol EC):
[0044] (1) Raw material ratio:
[0045] Percentage by weight, tebuconazole 30%, L-α-terpineol 10%, calcium dodecylbenzenesulfonate 4%, alkylaryl polyoxypropylene polyoxyethylene ether 6%, acetone added to 100%.
[0046] (2) Preparation method:
[0047] Add tebuconazole, L-α-terpineol, calcium dodecylbenzenesulfonate, and alkylaryl polyoxypropylene polyoxyethylene ether into acetone in sequence and stir until a homogeneous liquid is obtained, and the active ingredient content is 40%. Composition EC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com