Roxatidine acetate hydrochloride used for injection and preparation method thereof
A technology for roxatidine hydrochloride acetate and injection, which is applied in the field of preparation of roxatidine hydrochloride acetate for injection, can solve the problems of affecting product quality, instability to light, unfavorable storage, etc., and achieve the effect of metabolism in vivo Small, strong gastric acid secretion inhibitory effect, easy to store
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
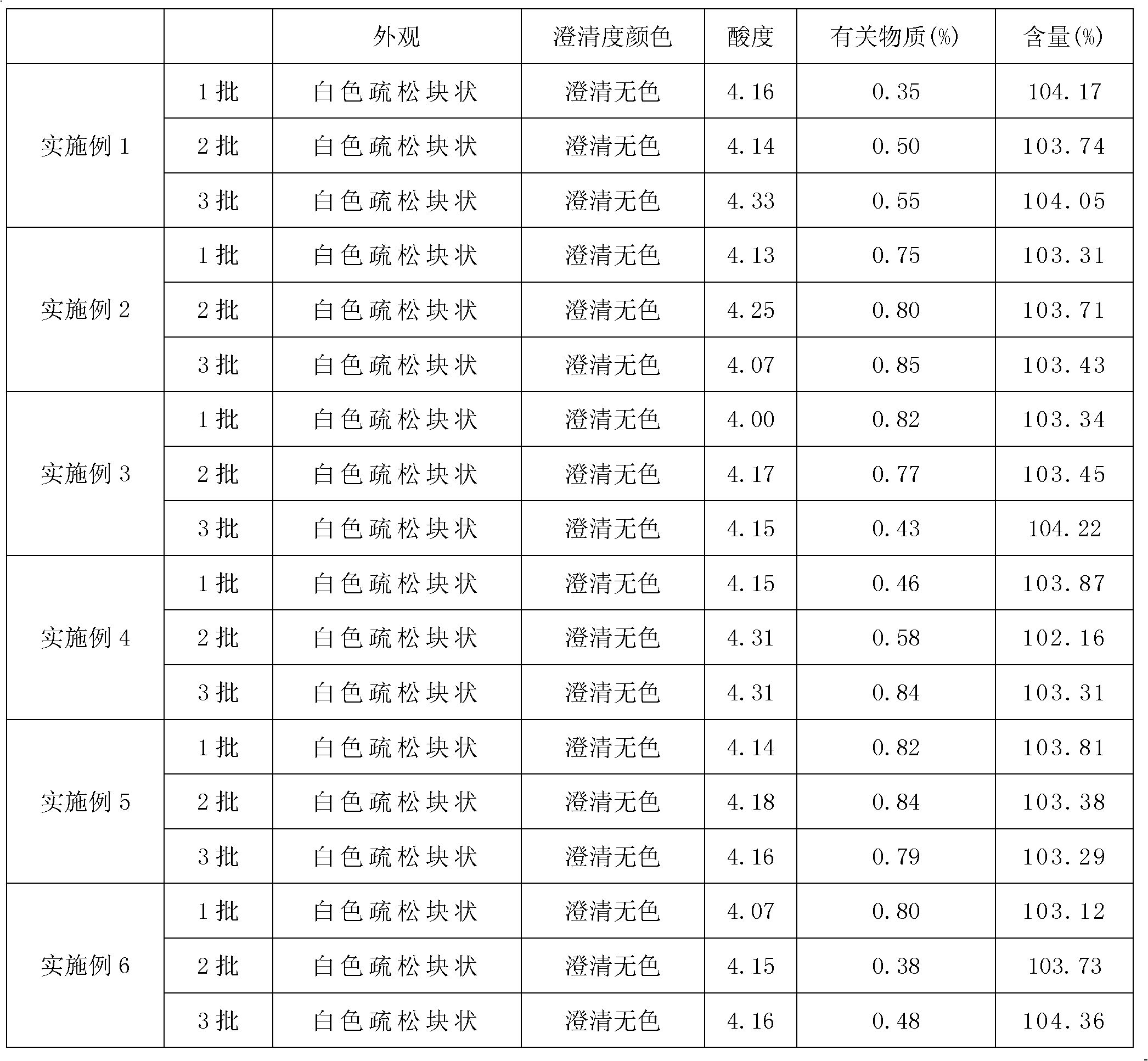
Embodiment 1
[0026] prescription:
[0027] Roxatidine Hydrochloride Acetate 75g
[0028] Glycine 65g
[0029] Add water for injection to 2000ml
[0030] Made 1000 pieces
[0031] Process:
[0032] (1) Weigh Roxatidine Hydrochloride Acetate and Glycine according to the prescription ratio and mix evenly, add 1500ml of water for injection therein, stir to dissolve completely, add activated carbon, stir for 10 minutes, filter, and decarbonize to obtain System A.
[0033] (2) Add 2 mol / L hydrochloric acid solution dropwise to A while stirring, and adjust the pH to 3.0-5.0 to obtain system B.
[0034] (3) Add water for injection to B to the full amount, and stir evenly to obtain system C.
[0035] (4) After the C semi-finished product passes the inspection, it is filtered through a 0.22 μm microporous membrane in a 100-class laminar flow ultra-clean room according to the aseptic operation requirements, and then packed into 7ml control antibiotic bottles with a ventilated rubber stopper.
...
Embodiment 2
[0038] prescription:
[0039] Roxatidine Hydrochloride Acetate 75g
[0040] Mannitol 65g
[0041] Add water for injection to 2000ml
[0042] Made 1000 pieces
[0043] Process:
[0044] (1) Weigh roxatidine hydrochloride acetate and mannitol according to the prescription ratio and mix evenly, add 1500ml of water for injection therein, stir to dissolve completely, add activated carbon, stir for 10 minutes, filter, and decarbonize to obtain system A.
[0045] (2) Add 2 mol / L hydrochloric acid solution dropwise to A while stirring, and adjust the pH to 3.0-5.0 to obtain system B.
[0046] (3) Add water for injection to B to the full amount, and stir evenly to obtain system C.
[0047] (4) After the C semi-finished product passes the inspection, it is filtered through a 0.22 μm microporous membrane in a 100-class laminar flow ultra-clean room according to the aseptic operation requirements, and then packed into 7ml control antibiotic bottles with a ventilated rubber stopper. ...
Embodiment 3
[0050] prescription:
[0051] Roxatidine Hydrochloride Acetate 75g
[0052] Glycine 75g
[0053] Add water for injection to 2000ml
[0054] Made 1000 pieces
[0055] Process:
[0056] (1) Weigh Roxatidine Hydrochloride Acetate and Glycine according to the prescription ratio and mix evenly, add 1500ml of water for injection therein, stir to dissolve completely, add activated carbon, stir for 10 minutes, filter, and decarbonize to obtain System A.
[0057] (2) Add 2 mol / L hydrochloric acid solution dropwise to A while stirring, and adjust the pH to 3.0-5.0 to obtain system B.
[0058] (3) Add water for injection to B to the full amount, and stir evenly to obtain system C.
[0059] (4) After the C semi-finished product passes the inspection, it is filtered through a 0.22 μm microporous membrane in a 100-class laminar flow ultra-clean room according to the aseptic operation requirements, and then packed into 7ml control antibiotic bottles with a ventilated rubber stopper.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com