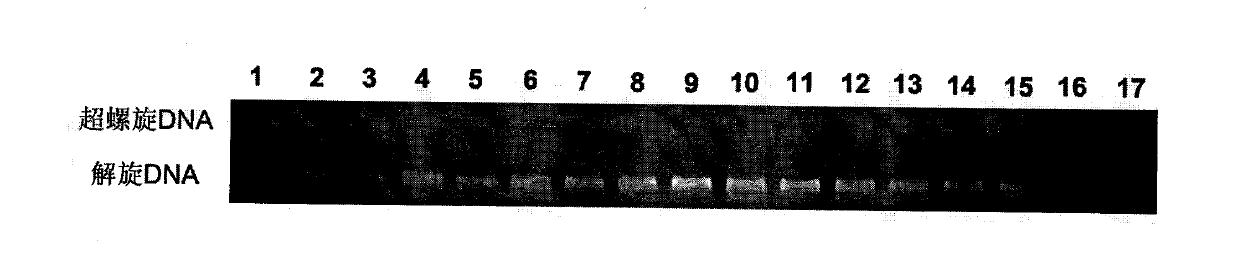
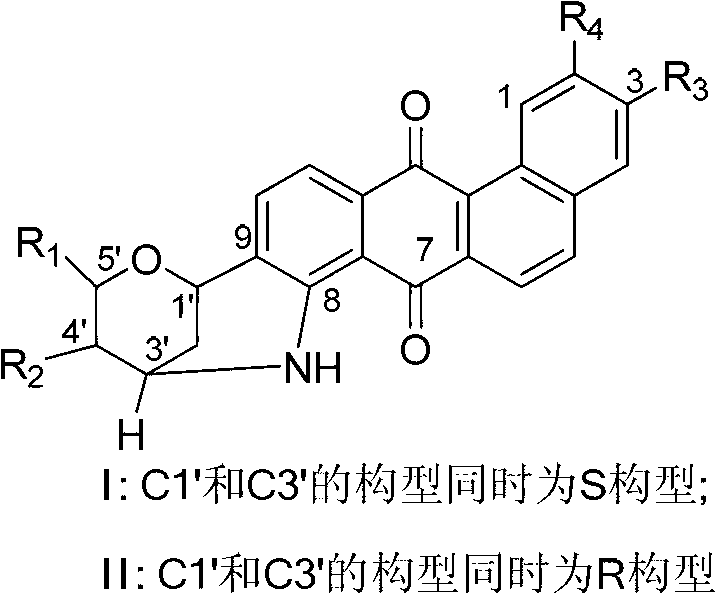
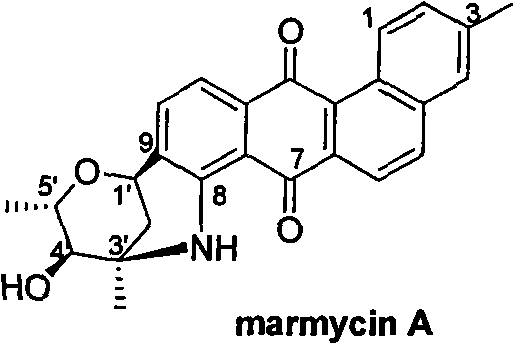
Novel benzanthracene cyclic compounds and preparation method and use thereof
A technology of benzanthracene and compounds, which is applied in the field of benzanthracycline compounds, can solve the problems of difficult synthesis, difficult derivatization, and no report on the synthesis of MarmycinA, etc., and achieve the effect of short reaction steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1L-rhamnocene is the preparation of the glycosylation product Ia-1, Ia-2 and IIa-1, IIa-2 of the sugar moiety
[0052] Step 1: 2-Bromo-5-acetoxy-1,4-p-naphthoquinone (compound 1)
[0053] Dissolve 1,5-diacetoxynaphthalene (1220mg, 5mmol) in 50mL glacial acetic acid, heat to dissolve it, then weigh NBS (3560mg, 20mmol) and dissolve it in 50mL glacial acetic acid and 100mL water, use a dropping funnel The solution of glacial acetic acid and water of NBS was added dropwise to the glacial acetic acid solution of the reactant, and stirred while dropping, for a total of 5 minutes. During the dropwise addition, a yellow solid was produced, and then the reaction solution was stirred at 65° C. for 2 hours. After the reaction, add 100 mL of water to the reaction solution, then extract three times with ethyl acetate, combine the organic phases, wash twice with saturated sodium bicarbonate solution, then wash with saturated brine, dry over anhydrous sodium sulfate, spin ...
Embodiment 2
[0094] Example 2D-Glucosene is the glycosylation product Ib-1, IIb-1 and Ib-2, IIb-2 of the sugar moiety
[0095]
[0096] The acetylated glycosidation products Ib-1 and IIb-1 and the deacetylated Glycosidation products Ib-2 and lib-2.
[0097] Acetylated glycosidation product Ib-1, dark red solid, 1 H-NMR (300MHz, CDCl 3 ): δ9.80(d, 1H, J=3.9Hz), 9.54(d, 1H, J=8.7Hz), 8.33(d, 1H, J=8.7Hz), 8.08(d, 1H, J=8.4Hz ), 7.65(s, 1H), 7.55(m, 3H), 4.90(m, 2H), 4.18(m, 2H), 4.06(dd, 1H, J=2.1, 12.0Hz), 3.61(ddd, 1H, J=2.4, 4.2, 10.2Hz), 2.54(s, 3H), 2.38(dd, 1H, J=1.5, 12.0Hz), 2.16(s, 3H), 2.06(s, 3H), 2.01(m, 1H ); 13 C-NMR (75MHz, CDCl 3 ): δ186.2, 185.6, 170.8, 170.1, 148.3, 138.7, 136.8, 136.5, 136.2, 134.6, 134.4, 132.1, 128.7, 128.4, 128.2, 127.6, 126.1, 122.3, 115.9, 111.7, 9.5 62.9, 46.2, 27.2, 21.5, 21.1, 20.8; MS (EI-LR) 499 (M+).
[0098] Acetylated glycosidation product IIb-1, dark red solid, 1 H-NMR (300MHz, CDCl 3 ): δ10.04(d, 1H, J=5.4Hz), 9.55(d, 1H, J=9Hz)...
Embodiment 3
[0101] Example 3D-Galactosene is the glycosylation product Ic-1, IIc-1 and Ic-2, IIc-2 of the glycan
[0102]
[0103] The acetylated glycosidation products Ic-1 and IIc-1 were prepared according to Step 9 of Example 1, except that the acetylated L-rhamnosene was replaced by acetylated D-galactosene 13 and deacetylated The glycosylation products Ic-2 and IIc-2.
[0104] Acetylated glycosidation product Ic-1, dark red solid, 1 H-NMR (300MHz, CDCl 3 ): δ9.90(d, 1H, J=5.1Hz), 9.53(d, 1H, J=8.7Hz), 8.32(d, 1H, J=8.1Hz), 8.07(d, 1H, J=8.7Hz ), 7.65(s, 1H), 7.58(m, 3H), 4.93(br s, 1H), 4.80(br s, 1H), 4.18(dd, 1H, J=6.0, 11.1Hz), 4.04(m, 2H), 3.77(m, 1H), 2.56(m, 4H), 2.16(s, 3H), 1.96(s, 3H), 1.70(d, 1H, J=13.2Hz). MS(EI-LR): 499(M + ).
[0105] Acetylated glycosidation product IIc-1, dark red solid, 1 H-NMR (300MHz, CDCl 3 ): δ9.82(d, 1H, J=5.1Hz), 9.55(d, 1H, J=9.0Hz), 8.33(d, 1H, J=8.4Hz), 8.07(d, 1H, J=9.0Hz ), 7.64(s, 1H), 7.55(q, 3H, J=7.8, 17.7Hz), 5.10(t, 1H, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com