Method for preparing 2-(1,3-dioxo-1,3-dihydro-isoindol-2-yl) glutaric acid-5-benzyl ester
A technology of dihydroisoindole and glutaric acid, applied in the preparation of glutamic acid derivatives, the synthesis of N-phthaloylglutamic acid-γ-benzyl ester, compound 2-glutaric acid-5 - In the field of benzyl esters, it can solve the problems of relatively expensive phthalimide, low yield of benzyl glutamate, uneconomical preparation and use, and achieve novel synthetic routes and methods, cheap raw materials, and good chirality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
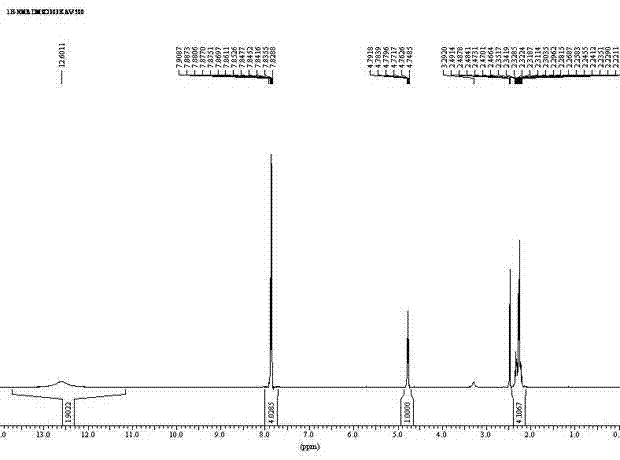
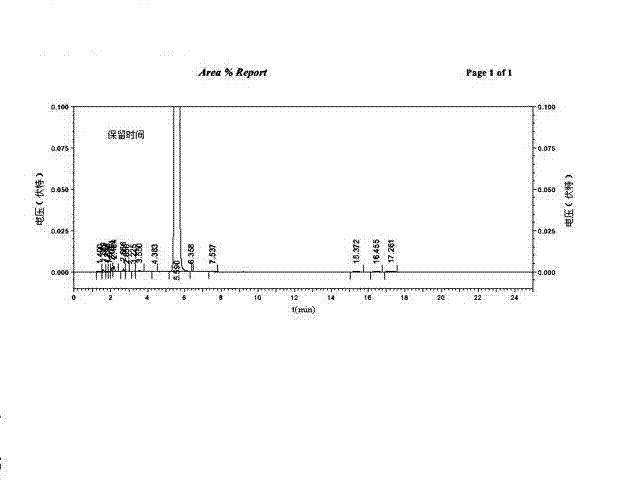
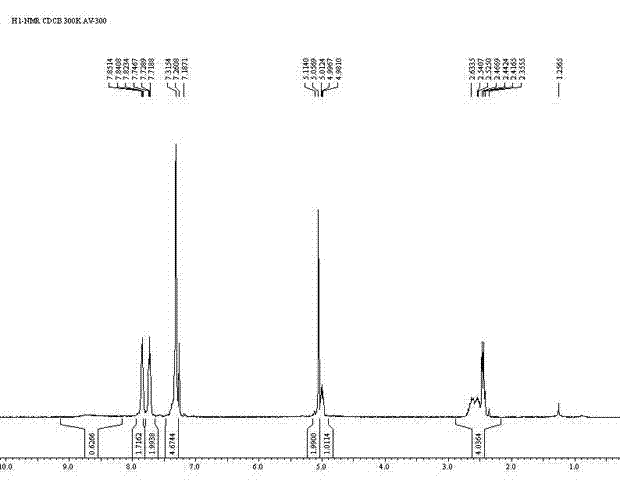
[0036] Put L-glutamic acid (14.72g, 0.1mol) in the 500ml flask, phthalic anhydride (14.82g, 0.1mol), toluene (300ml) and triethylamine (7.2ml, 0.05mol), be equipped with reflux separator, Stir and heat to reflux for 7 hours, and after cooling, remove the solvent by rotary evaporation at 50°C, add 1N HCl (40ml) and stir thoroughly, then add dichloromethane (60ml), stir to precipitate a large amount of solid, filter, and wash the filter cake with water and dichloromethane successively. Vacuum drying gave Intermediate 1 as a white solid N-phthaloyl-L-glutamic acid (21.87 g), with a yield of 78.9%.
[0037] Intermediate 1 (21.87g, 0.079mol) prepared above was put into a 250ml flask, then added benzyl alcohol (17g, 0.157mol), acetone (50ml) and p-toluenesulfonic acid (2.7g, 0.0157mol), heated to reflux for 3 hour, after cooling, rotary evaporation removed the solvent, added dichloromethane (40ml) and stirred for 2 hours, filtered off the solid, and the solid was washed with dichlor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com