Indolinone compound
A compound, the technology of -CON, applied in the field of indolinone compounds, can solve the problem of no disclosure and suggestion of TRPA1 channel activation or digestive system function improvement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
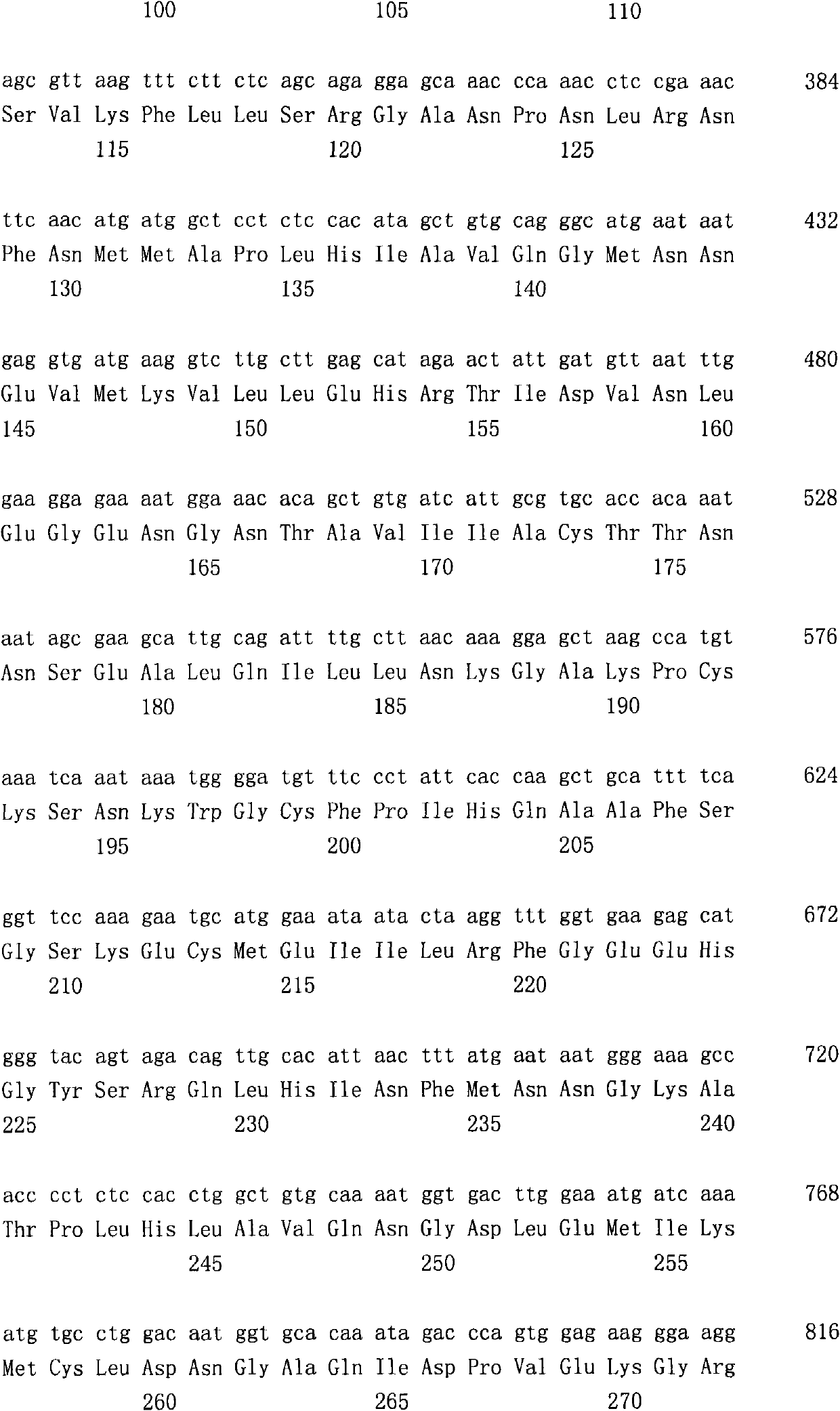
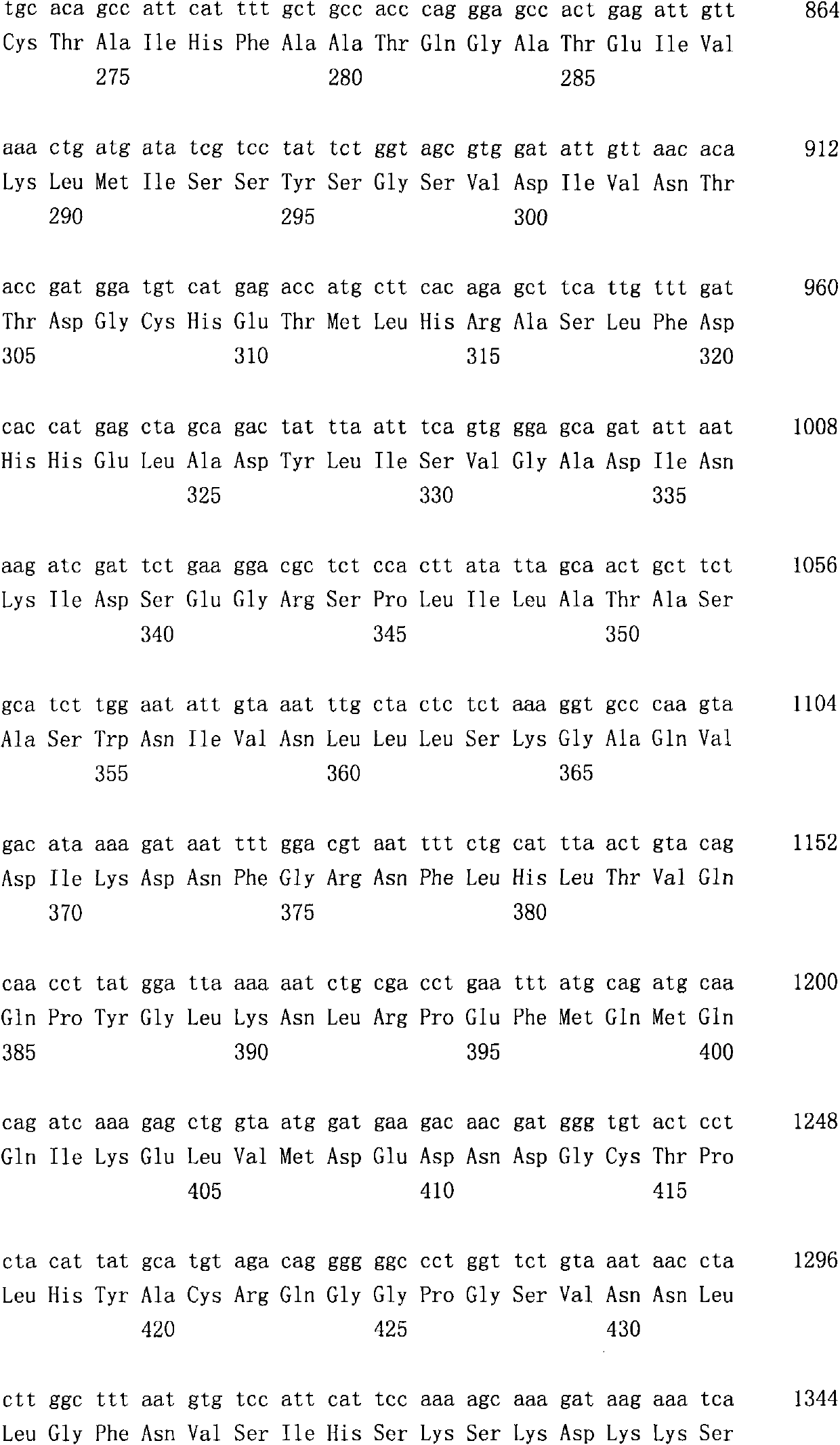
[0274] Dissolve 1.651g 7-fluoro-1H-indole-2,3-dione in 10mL DMF, add 600mg 60% sodium hydride oil solution under ice-cooling, stir for 30 minutes, then add 1.10mL 1-bromo-2- Methylpropane, stirred for 4 hours. The solvent was distilled off under reduced pressure, and the residue was diluted with ethyl acetate and washed successively with water and saturated brine. The organic layer was dried over anhydrous magnesium sulfate, and then the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography (hexane:ethyl acetate=90:10) to obtain 1.032 g of 7-fluoro-1-isobutyl -1H-indole-2,3-dione.
preparation example 2
[0276] Dissolve 600mg of 1,3-dihydro-2H-indol-2-one in 15mL of EtOH, add 794mg of 1-methyl-1H-imidazole-2-carbaldehyde (Calvarde) and 0.06mL of piperidine, at 75°C Stir for 1 hour. The precipitated solid was collected by filtration to obtain 1.180 g of 3-[(1-methyl-1H-imidazol-2-yl)methylene]-1,3-dihydro-2H-indol-2-one.
preparation example 3
[0278] 1.027g of 7-fluoro-1-isobutyl-1H-indole-2,3-diketone was dissolved in 10mL THF, and 1.779g (triphenylphosphorous) ethyl acetate ((Trifenylphosphorous) ethyl acetate ( Liden) acetic acid Echel), stirred at 60°C for 10 hours. The solvent was distilled off under reduced pressure, and the residue was dissolved in ethyl acetate, and washed successively with saturated sodium bicarbonate water and saturated brine. The organic layer was dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography (hexane:ethyl acetate=90:10) to obtain 724 mg of (2E)-(7-fluoro-1 - ethyl isobutyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)acetate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com