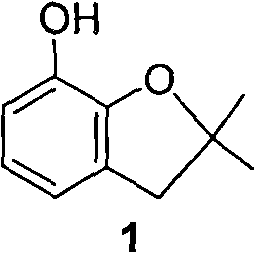
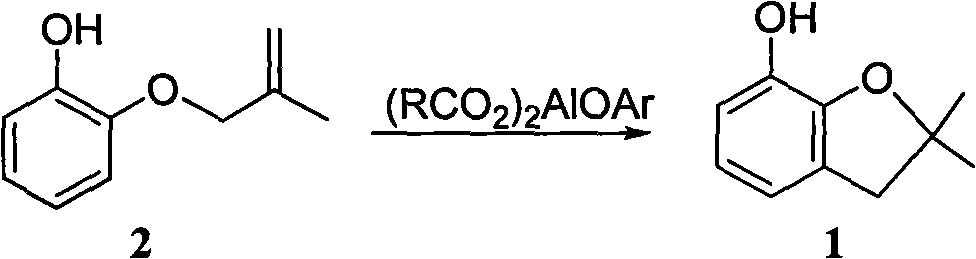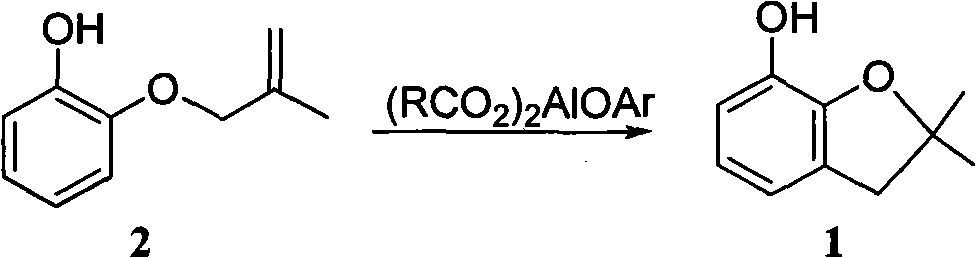Method for synthesizing furan phenol by using aluminum dicarboxylate phenol as catalyst
A technology for catalyzing and catalyzing aluminum phenate dicarboxylate is applied in the field of preparation of furan phenol, and can solve the problems of not being able to control the reaction temperature and reduce the selectivity, so as to improve the selectivity of the cyclization reaction and improve the yield. efficient, easy to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0021] Embodiment 1 Diacetate aluminum phenate catalyzes the synthesis of furan phenol
[0022] (1) Preparation of Aluminum Diacetate Phenolate
[0023]
[0024] 20 mL of o-dichlorotoluene, 0.01 mol of aluminum isopropoxide and 0.01 mol of phenol, stirred at 35° C. for 1 h, rotary-evaporated to recover isopropanol, and cooled to obtain aluminum phenate diacetate.
[0025] (2) Catalytic synthesis of furanol with aluminum phenate diacetate
[0026]
[0027] 0.01mol aluminum phenate diacetate, 164g 2-(2-methallyloxy)phenol and 100mL o-dichlorobenzene were reacted at 180°C for 3.0h; after post-treatment, furan phenol was obtained in a yield of 82.6% (liquid chromatography, external standard, in terms of 2-(2-methylallyloxy)phenol, the same below).
Embodiment 2 2
[0028] Embodiment 2 2-methoxy phenol aluminum diacetate catalyzes the synthesis of furan phenol
[0029] (1) Preparation of 2-methoxy phenol aluminum diacetate
[0030]
[0031] 25 mL of N-methylpyrrolidone and toluene, 0.01 mol of aluminum isopropoxide acetate and 0.01 mol of 2-methoxyphenol, stirred at 40° C. for 1 h, rotary-evaporated to recover isopropanol, and cooled to obtain 2-methoxy phenolic aluminum diacetate.
[0032] (2) 2-Methoxy phenol aluminum diacetate catalyzed synthesis of furan phenol
[0033]
[0034] 0.01mol of 2-methoxyaluminum diacetate, 164g of 2-(2-methylallyloxy)phenol and 100mL of N-methylpyrrolidone were reacted at 165°C for 5.5h; after post-treatment, furanol was obtained with a yield of 82.3%.
Embodiment 3 2
[0035] Embodiment 3 2-naphthol aluminum diacetate catalyzes the synthesis of furanol
[0036] (1) Preparation of 2-naphthol aluminum diacetate
[0037]
[0038] 20 mL of xylene and diethylene glycol monomethyl ether, 0.01 mol of aluminum isopropoxide acetate and 0.01 mol of 2-naphthol, stirred at 35°C for 1 h, rotary-evaporated to recover isopropanol, and cooled to obtain 2-naphthol aluminum diacetate.
[0039] (2) 2-Naphthol aluminum diacetate catalyzed synthesis of furanol
[0040]
[0041] 0.01mol 2-naphthol aluminum diacetate, 164g 2-(2-methylallyloxy)phenol and 80mL diethylene glycol monomethyl ether were reacted at 180°C for 4.5h; after post-treatment, furan phenol was obtained with a yield of 82.0% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com