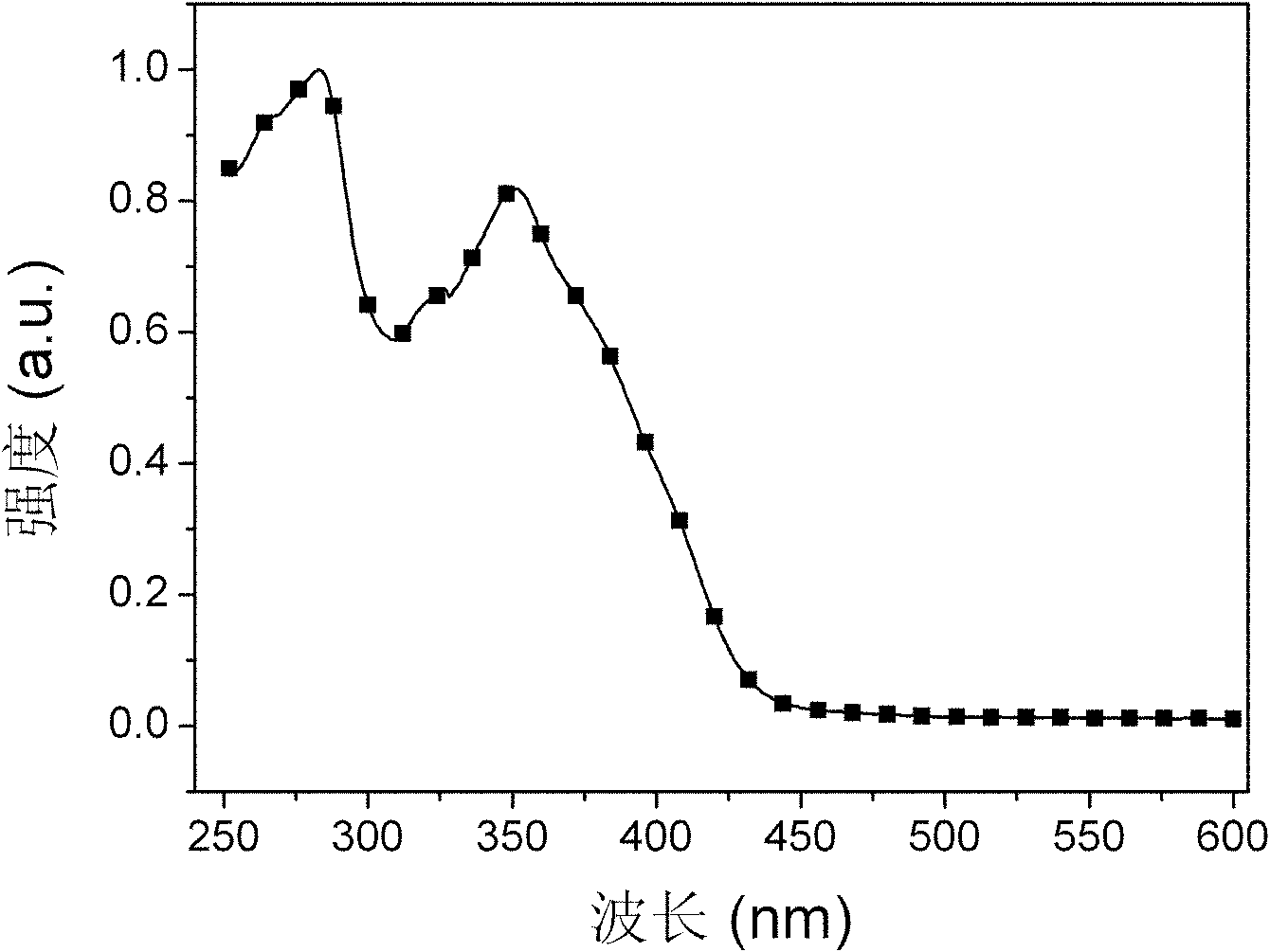
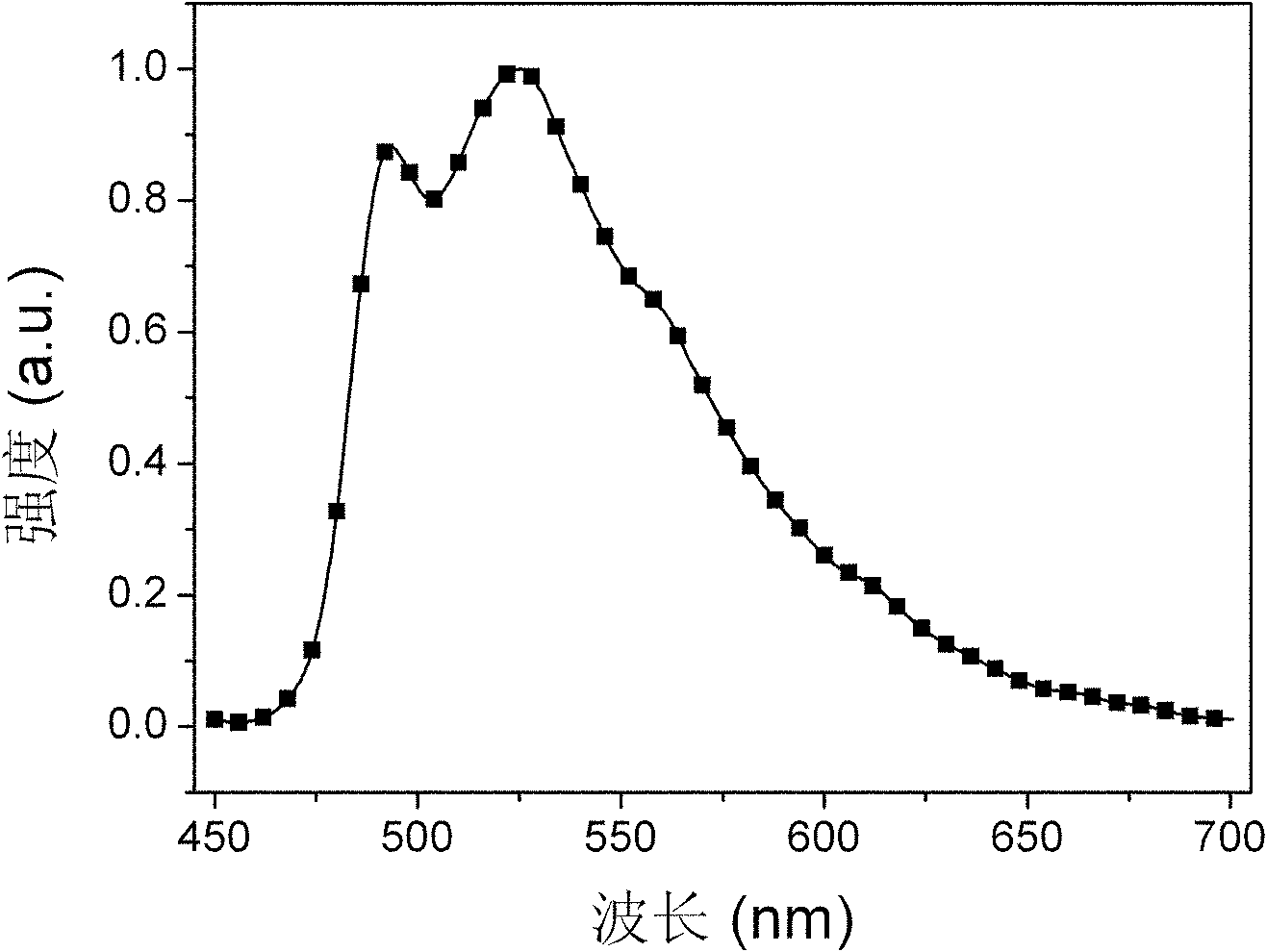
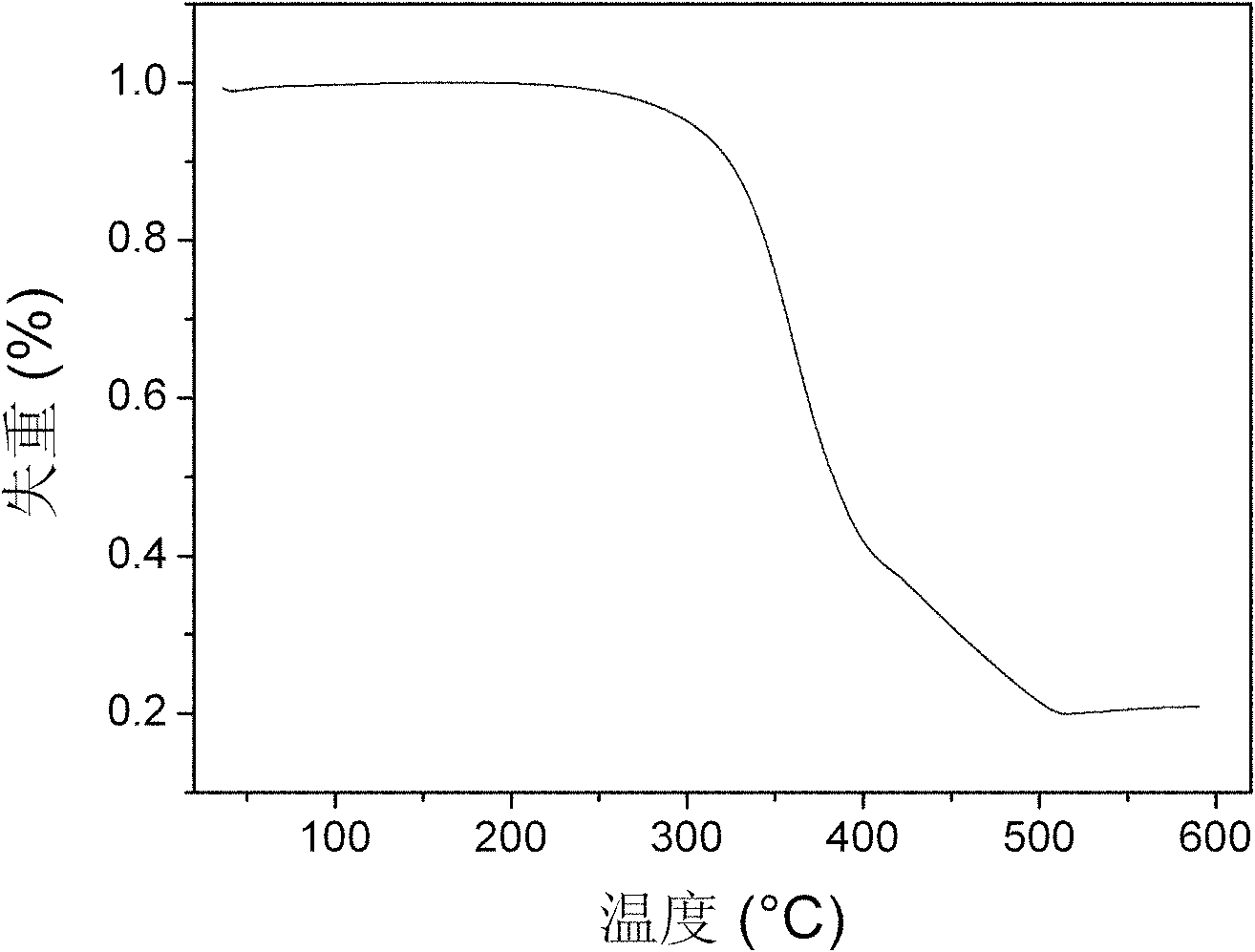
Bi(phenylpyridine) fluorene derivatives and binuclear liquid crystal polarized cyclometalated platinum complex
A platinum complex, pyridine phenyl technology, applied in the field of liquid crystal polarized organic light-emitting materials, can solve the problem of low luminous efficiency and luminous brightness of liquid crystal polarized OLEDs, restricting the development and application of liquid crystal polarized light-emitting diodes, and disadvantageous high-efficiency, high-brightness liquid crystals problems such as backlight source, to achieve the effect of easy synthesis and purification, easy product, good liquid crystal performance and polarized luminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of 2-(4'-bromophenyl)-5-(alkoxymethyl)pyridine
[0048]
[0049] 1.1 Synthesis of 2-(4'-bromophenyl)-5-(butoxymethyl)pyridine
[0050] In a 100mL three-necked flask, add 5.0g (25mmol) 4-bromophenylboronic acid ester, 6.1g (25mmol) 2-bromo-5-(butoxymethyl)pyridine, 0.58g (0.5mmol) Pd(PPh 3 ) 4 , 15mL 2M K 2 CO 3 Solution, 30mL of toluene and 15mL of ethanol were reacted at 80°C for 24h under the protection of nitrogen. After cooling, the reaction solution was poured into saturated NH 4 Cl solution, extracted with dichloromethane, the extract was washed with water, anhydrous Na2SO 4 After drying, distilling off the solvent, and column separation (petroleum ether / ethyl acetate=20:1), 6.8 g of white solid was obtained. Yield 85.6%. 1 H NMR (400MHz, CDCl 3 , TMS), 8.65 (s, 1H), 7.90-7.89 (d, J=8.4Hz, 2H), 7.78-7.76 (d, J=7.0Hz, 1H), 7.71-7.69 (d, J=8.1Hz, 1H), 7.62-7.60(d, J=8.4Hz, 2H), 4.57(s, 2H), 4.06-4.00(t, 2H), 3.55-3.52(t, 2H), 1.70-1.60(m, 2H) ...
Embodiment 2
[0056] Synthesis of 5-(alkoxymethyl)-2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)pyridine
[0057]
[0058] 2.1 Synthesis of 5-(butoxymethyl)-2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)pyridine
[0059] In a 100mL three-necked flask, add 2.0g (5.4mmol) 2-(4'-bromophenyl)-5-(butoxymethyl)pyridine, 1.5g (5.9mmol) bisanalyl borate, 1.6g (16.2mmol) potassium acetate, 0.13g (0.16mmol) PdCl 2 (dppf)CH 2 Cl 2 And 60mL of dimethyl sulfoxide, reacted at 80°C for 24h under the protection of nitrogen. Cool, pour the reaction solution into 100mL water, extract with dichloromethane, wash the extract with water, anhydrous Na 2 SO 4 Drying, distillation to remove the solvent, and column separation (petroleum ether / ethyl acetate=10:1) yielded 1.2 g of off-white solid with a yield of 60.5%. 1 H NMR (400MHz, CDCl 3 , TMS), δ (ppm): 8.67 (s, 1H), 8.03-8.01 (d, J = 8.0Hz, 2H), 7.94-7.92 (d, J = 8.0Hz, 2H), 7.78 (s, 2H) , 4.57(s, 2H), 3.54-3.50(t, 2H), 1.67-1....
Embodiment 3
[0065] Synthesis of 2,7-bis[4'-(4"-alkoxymethyl-2"-pyridine)phenyl]-9,9-bis(dodecyl)fluorene
[0066]
[0067] 3.1 Synthesis of 2,7-bis[4′-(4″-butoxymethyl-2″-pyridine)phenyl]-9,9-bis(dodecyl)fluorene
[0068] In a 100mL three-necked flask, add 1.0g (2.7mmol) 2-[(4′-bromophenyl)-5-(alkoxymethyl)pyridine]-4,4,5,5-tetramethyl-1, 3,2-Dioxaborane, 0.8g (1.2mmol) 9,9-dioctyl-2,7-dibromofluorene, 93mg (0.08mmol) Pd(PPh 3 ) 4 , 15mL 2M K 2 CO 3 Solution, 30mL of toluene and 15mL of ethanol were reacted at 80°C for 24h under the protection of nitrogen. After cooling, the reaction solution was poured into saturated NH 4 Cl solution, extracted with dichloromethane, the extract was washed with water, anhydrous Na 2 SO 4 After drying, distilling off the solvent, and column separation (petroleum ether / ethyl acetate=10:1), 0.89 g of white solid was obtained after drying, with a yield of 75.6%. 1 H NMR (400MHz, CDCl 3 , TMS), 8.70(s, 2H), 8.15-8.13(d, J=8.2Hz, 4H), 7.83-7.81(m, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com