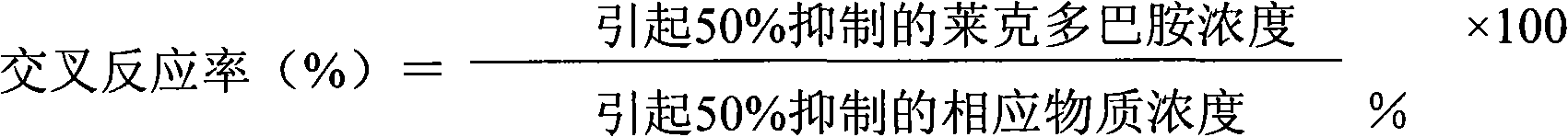Anti-ractopamine antibody and application thereof
A technology of ractopamine and single-chain antibody, which is applied in the application, use of vectors to introduce foreign genetic material, and cells modified by introducing foreign genetic material. Simple, sensitive, and easy-to-produce effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
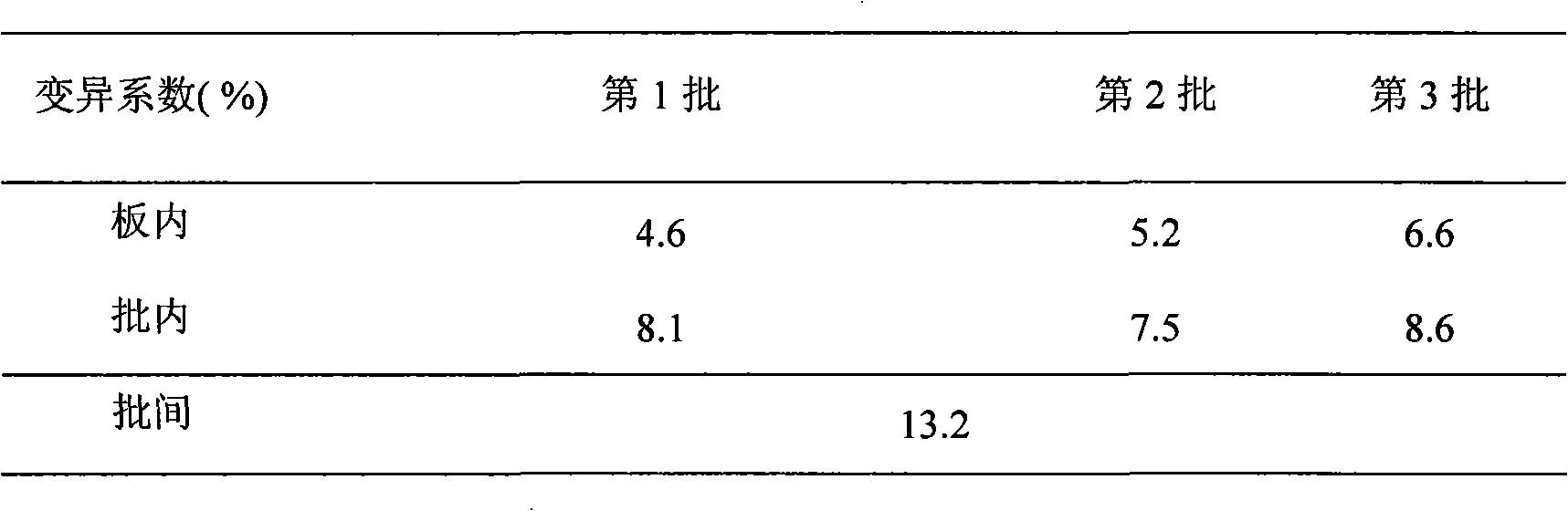
[0056] Example 1. Preparation and functional detection of antibodies
[0057] 1. Preparation of ractopamine single-chain antibody
[0058] (1) Screening of antibodies
[0059] Six-month-old male Balb / c mice were taken, and the total RNA of spleen cells was extracted by one-step Trizol method, purified to obtain mRNA, and then reverse transcribed to obtain cDNA. A full set of primers were used to amplify the heavy chain variable region (VH) and light chain variable region (VL) genes respectively with cDNA as the template. Splicing into a single-chain antibody gene (ScFv), then connecting the ScFv with the vector pCANTAB5E to obtain pCANTAB5E / ScFv, and transforming E. coli TG1 to obtain a mouse-derived non-immune single-chain antibody library. Phage particles with specific anti-multiple ractopamine single-chain antibodies were screened by ELISA, and their corresponding nucleotide sequences were obtained by sequencing.
[0060] The single-chain antibody is composed of a heavy ...
Embodiment 2
[0090] Example 2. ELISA kit for detecting ractopamine and preparation thereof
[0091] 1. The ELISA kit consists of the following substances:
[0092] 1. ELISA plate coated with ractopamine hydrochloride and carrier protein conjugate (RCT-BSA);
[0093] 2. Ractopamine antibody: the single-chain antibody described in Example 1. The concentration of the antibody working solution was 5.0 ng / mL, and the antibody working solution was obtained by diluting the purified antibody in Example 1 with the sample diluent;
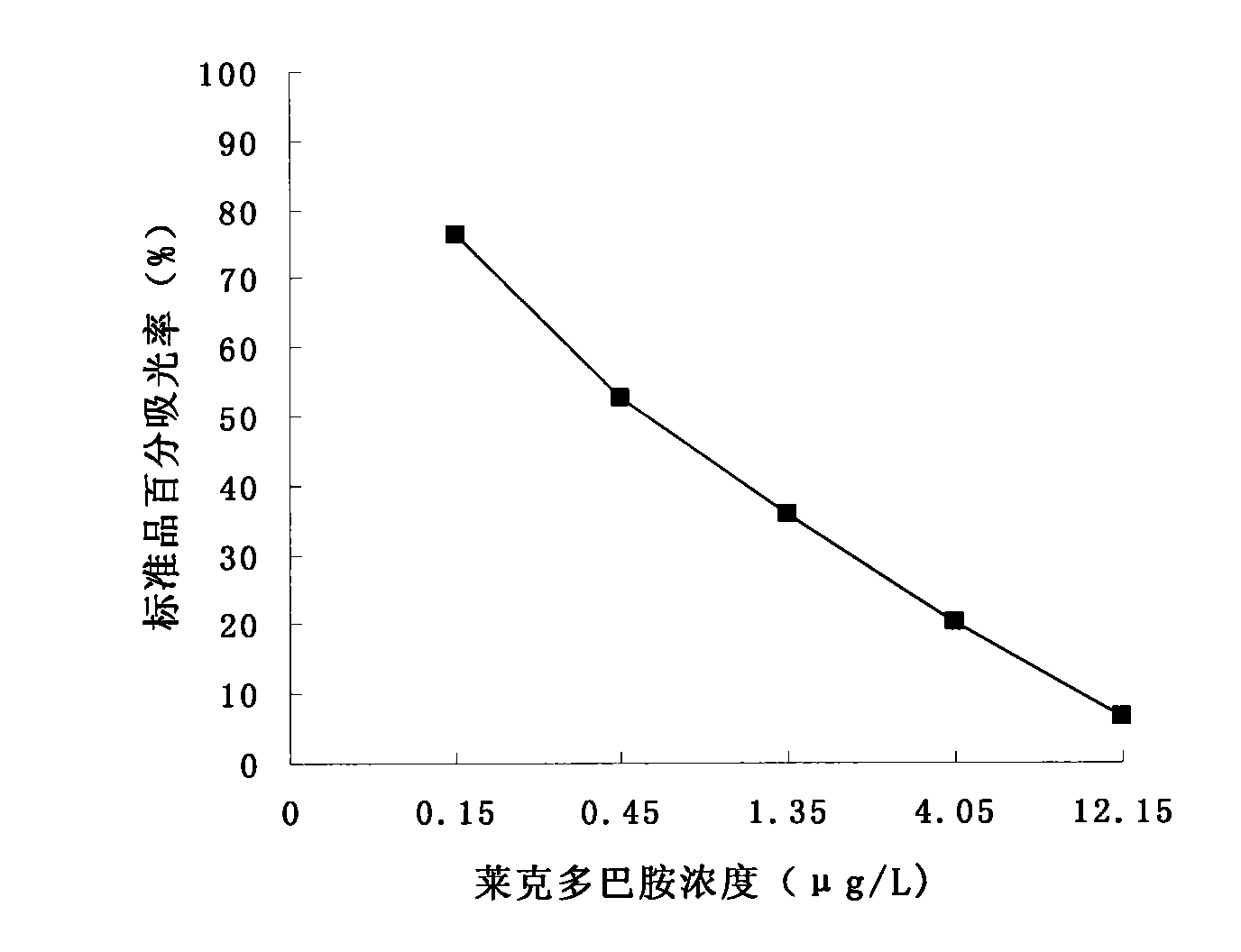
[0094] 3. Ractopamine standard: Ractopamine standard is ractopamine hydrochloride, the concentration of standard solution is 0μg / L, 0.15μg / L, 0.45μg / L, 1.35μg / L, 4.05μg / L and 12.15μg / L respectively ; Ractopamine hydrochloride was purchased from Sigma-Aldrich company in the United States; product catalog number is 34198; diluted to the above concentrations with sample diluent;
[0095] 4. Enzyme-labeled secondary antibody: horseradish peroxidase (HRP)-labeled mouse ant...
Embodiment 3
[0148] Embodiment 3, the test paper for detecting ractopamine and its preparation and application
[0149] 1. The structure of the test paper
[0150] The test paper is composed of a sample absorption pad, a colloidal gold pad, a reaction film and a water absorption pad;
[0151] The sample absorption pad, the colloidal gold pad, the reaction membrane and the water absorption pad are connected in sequence, the end of the sample absorption pad is connected to the beginning of the colloidal gold pad, the end of the colloidal gold pad is connected to the beginning of the reaction membrane, and the end of the reaction membrane is connected to the beginning of the colloidal gold pad. The beginning ends of the absorbent pads are connected;
[0152] The colloidal gold pad is coated with colloidal gold-labeled ractopamine antibody (the protein shown in sequence 1 in the sequence listing);
[0153] The reaction membrane has a detection area and a quality control area, and the detecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com