Anti-VEGF antibodies
An antibody and carrier technology, applied in the direction of antibodies, anti-inflammatory agents, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0323] Preparation of Antibody Drug Conjugates
[0324] In an antibody drug conjugate (ADC), an antibody is coupled to one or more drug moieties (D), eg, about 1 to about 20 drug moieties per antibody, via a linker (L). ADCs of general formula I can be prepared in several ways using organic chemistry reactions, conditions and reagents known to those skilled in the art, including: (1) reaction of the nucleophilic group of the antibody with a divalent linking reagent A valent bond forms Ab-L, which then reacts with the drug moiety D; and (2) the nucleophilic group of the drug moiety reacts with a divalent linker to form D-L via a covalent bond, which then reacts with the nucleophilic group of the antibody. Additional methods for preparing ADCs are described herein.
[0325] Ab-(L-D) p I
[0326] A linker may consist of one or more linker components. Exemplary linker components include 6-maleimidocaproyl ("MC"), maleimidopropanoyl ("MP"), valine-citrulline (" val-ci...
Embodiment 1
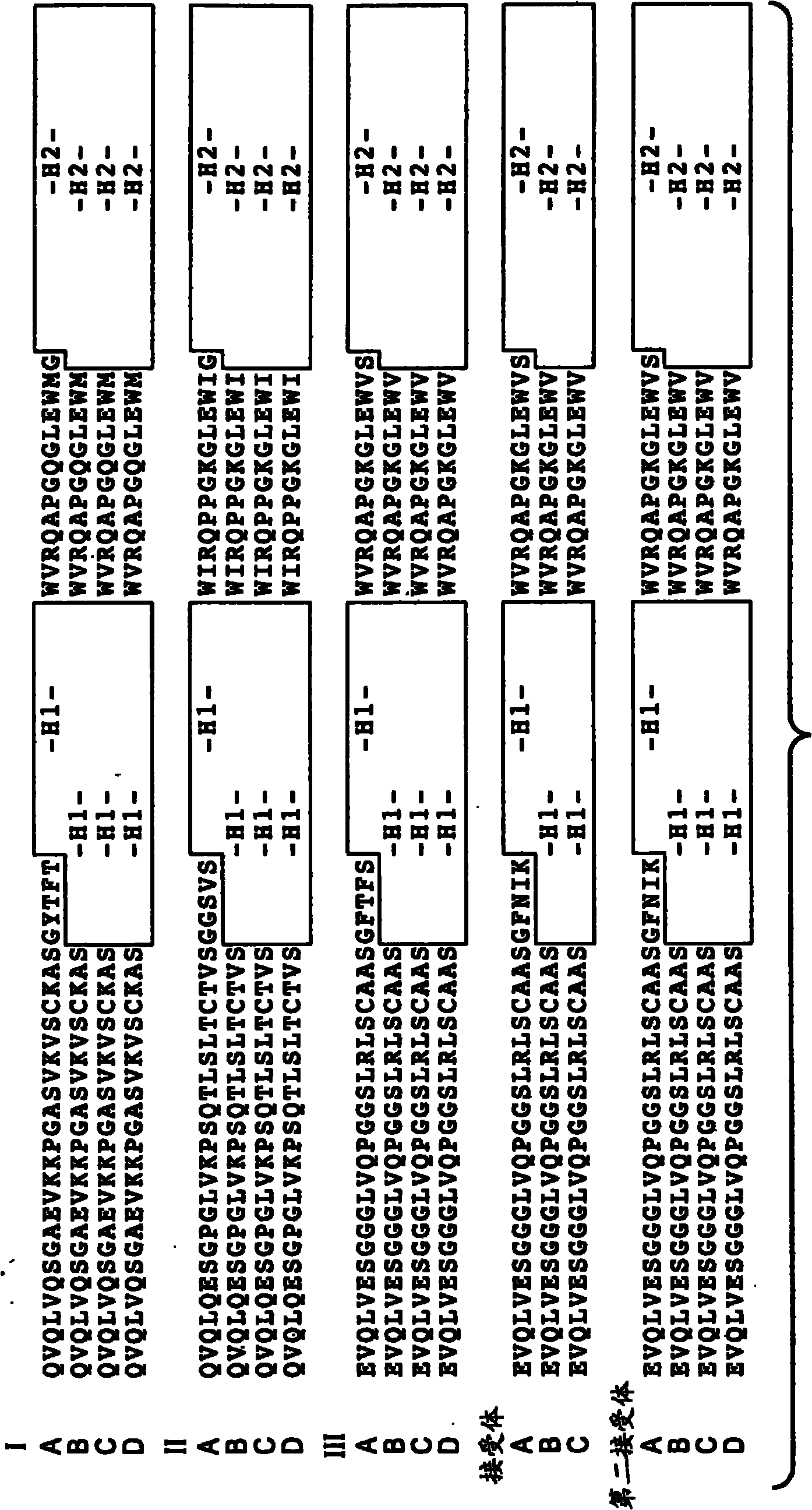
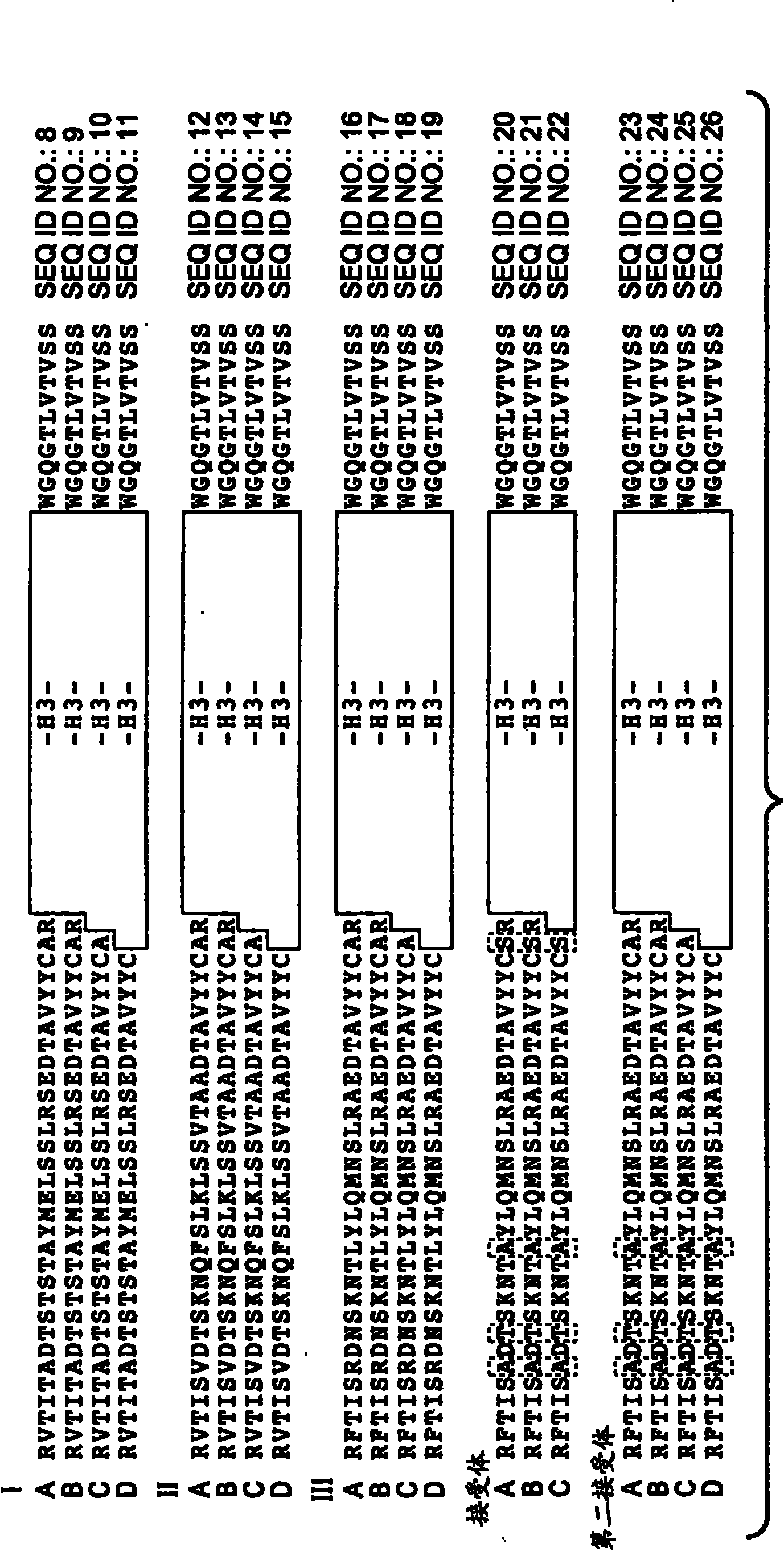
[0489] Generation and characterization of anti-VEGF antibodies
[0490] Construction of a synthetic phage antibody library (humanized anti-ErbB2 antibody, 4D5) on a single framework by introducing diversity in the complementarity determining regions (CDRs) of the heavy and light chains (Lee, C.V. et al. J Mol Biol 340 , 1073-93 (2004)). Briefly, a phage-displayed synthetic polypeptide library was constructed on a single human framework by introducing synthetic diversity at the solvent-exposed positions of the heavy chain complementarity determining regions (CDRs). To improve library performance, monovalent and bivalent antigen-binding fragment (Fab) libraries were constructed, and different CDR-H3 diversity was investigated by varying amino acid composition and CDR length. The library was then expanded by increasing the variability in CDR-H3 length and utilizing custom codons that mimic the amino acid composition of native CDR-H3 sequences. High affinity antibodies were gene...
Embodiment 2
[0493] Anti-VEGF Antibody Binding Affinity
[0494] To determine the binding affinity of anti-VEGF IgG, BIAcore TM The -3000 device performs Surface Plasmon Resonance (SRP) measurements. Carboxymethylated dextran biosensor chips (CM5, BIACORE, Inc.) were treated with N-ethyl-N'-(3-dimethylaminopropyl)-carbodiimide according to the supplier's instructions. (EDC) and N-hydroxysuccinimide (NHS) activation. Human or murine VEGF was immobilized to achieve approximately 60 response units (RU) of coupled protein. see Figure 10 . For kinetic measurements, two-fold serial dilutions of anti-VEGF IgG (7.8 nM to 500nM). Association rate (k on ) and dissociation rate (k off ) using the bivalent binding model (BIACORE Evaluation software, version 3.2) calculations. Calculate the equilibrium dissociation constant (Kd) as k off / k on ratio.
[0495] Since the off-rate of B20.4.1 was not measurable, IgG was immobilized to achieve approximately 1000 response units (RU) of coupled...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com