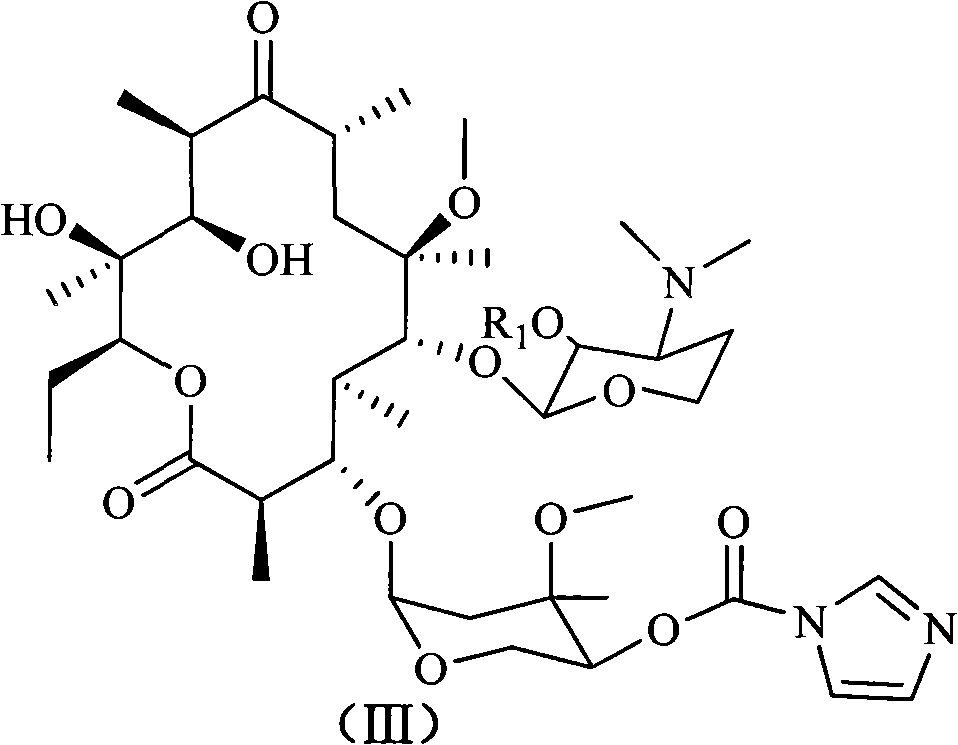
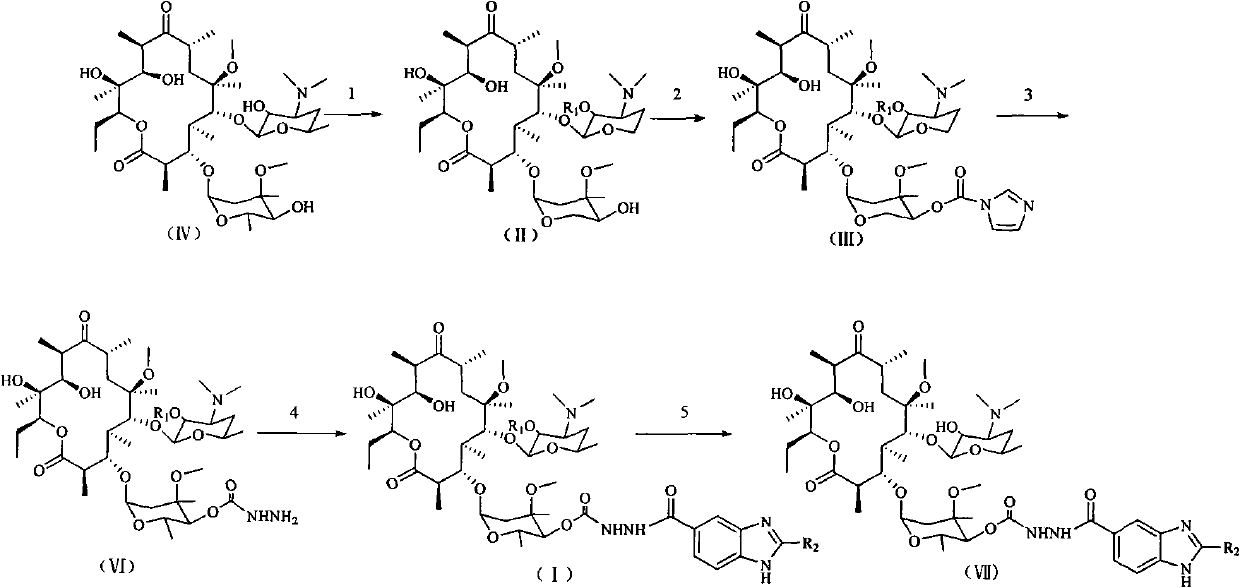
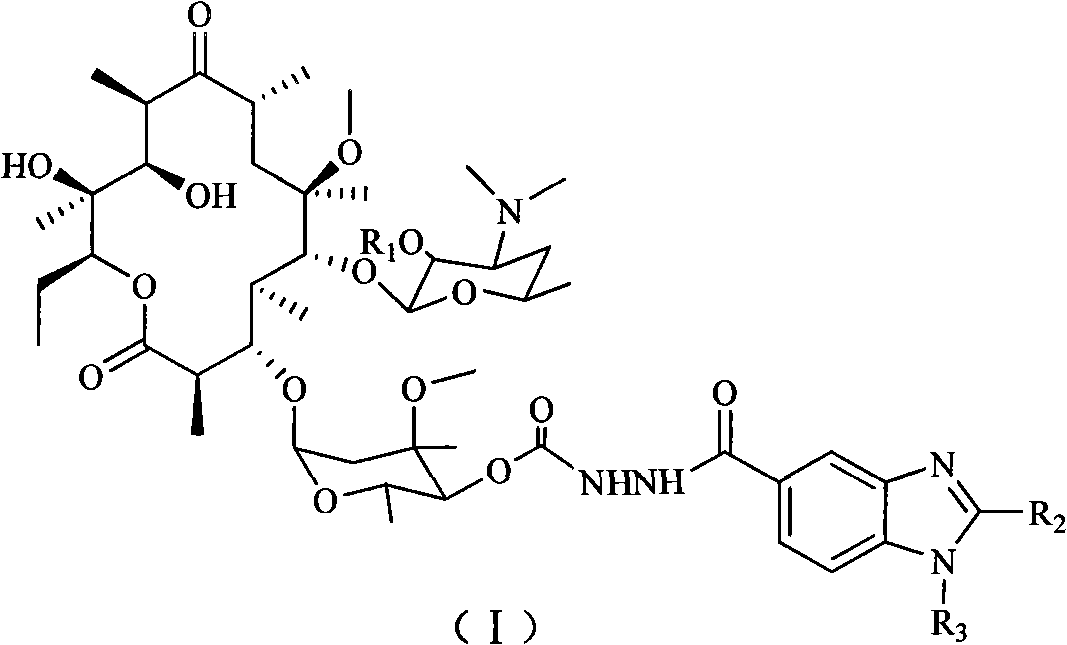
4''-benzimidazole-5-formacyl-ester carbazate clarithomycin derivative and midbody
A technology of clarithromycin carbamate and benzimidazole is applied in the field of 4″-benzimidazole-5-formyl-carbamate clarithromycin derivatives and intermediates, and can solve the ether bond Easy to oxidize, affect the binding of compound to target, easy to open ring, etc.
Inactive Publication Date: 2010-12-08
SHANDONG UNIV
View PDF13 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In addition, some 4″-OH modified clarithromycin derivatives have some unstable groups on the side chains, such as ester bonds are easily hydrolyzed in vivo or ether bonds are easily oxidized, etc., and some substituted heterocycles are in the Unstable in vivo and easy to open the ring, etc., will affect the combination of the compound and the target, thereby reducing the antibacterial activity
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a 4''-benzimidazole-5-formacyl-ester carbazate clarithomycin derivative shown in the formula (I) and a pharmaceutically-acceptable salt. R1 is hydrogen, acetyl or benzoyl; R2 is hydrogen, fatty alkyl, halogenated hydrocarbon or substituted aromatic radical; and R3 is hydrogen, fatty alkyl, halogen, halogenated hydrocarbon, benzyl or substituted benzyl. The invention also relates to a prepared intermediate product and a preparation method thereof, a pharmaceutically-acceptable salt formed with inorganic or organic acid, a medicine composition and application to treating bacterial infection. The derivative represents relatively strong in-vitro antibacterial activity to sensitive staphylococcus aureus, sensitive streptococcus pneumoniae and sensitive streptococcus pyogenes and represents obviously enhanced antibacterial activity to drug-resistant streptococcus pneumoniae derivated by different drug-resistant genes.
Description
technical field The invention relates to a 4″-benzimidazole-5-formyl-carbazate clarithromycin derivative, a preparation method thereof, and an intermediate. Background technique Macrolide antibiotics, as an important class of anti-infective drugs, have become second only to β-lactams in clinical application because of their good antibacterial activity, no allergic reactions, few side effects, and high safety. Antibiotics are the second largest class of anti-infective drugs and play an important role in clinical treatment. As the first generation of macrolide antibiotics, erythromycin has been widely used to treat infections caused by Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus hemolyticus and Mycoplasma pneumoniae, especially for those who are allergic to penicillin. However, its application is limited due to its instability to acid media and low bioavailability. The second-generation macrolide antibiotics represented by clarithromycin have solved this p...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07H17/08C07H1/00A61K31/7056A61P31/04
Inventor 马淑涛马陈晨齐昀坤马晓东
Owner SHANDONG UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com