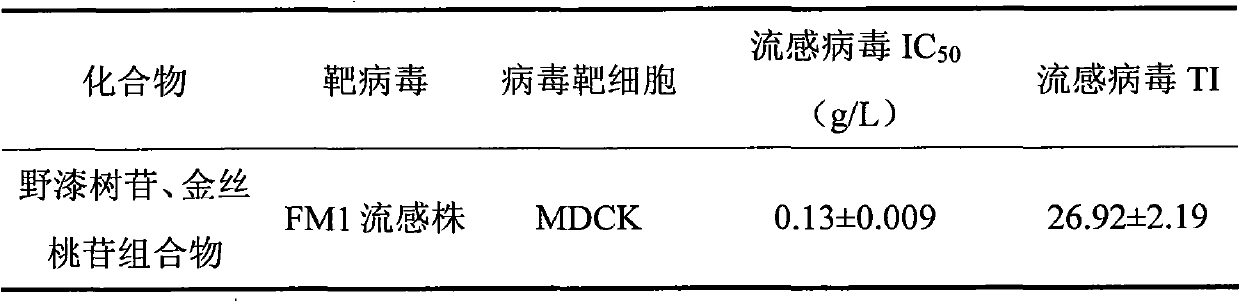
Rhoifolin and hyperin composition and application of medicines prepared therefrom
A technology of Rhodorin and Hyperoside, which is applied in the field of Rhodorin and Hyperin composition and its preparation of medicines, can solve problems such as undiscovered, and achieve inhibition of replication, reduction of infection, prevention and treatment of influenza and The effect of its complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Rhodorin and hyperin compositions of the present invention, wherein the molecular formula of rhodorin: C 27 h 30 o 14 , Molecular weight: 578.52, the structural formula of described Rhodorin is as follows:
[0031]
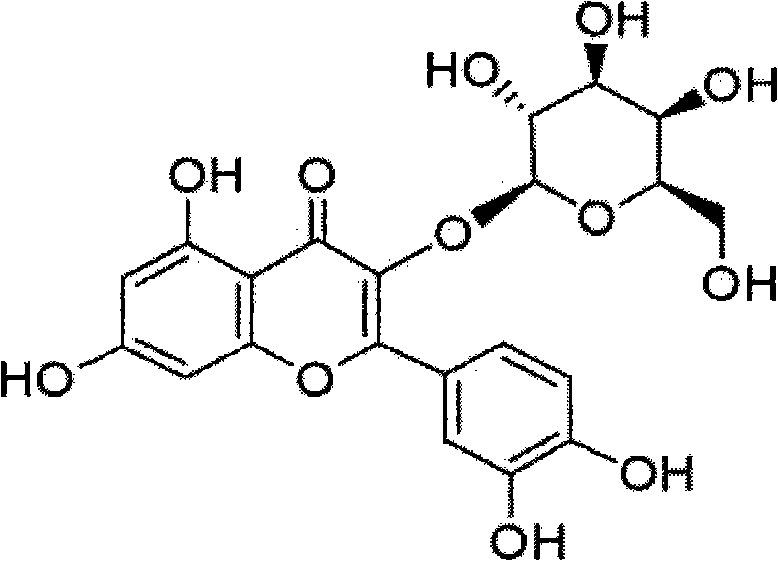
[0032] The molecular formula of the hyperin: C 21 h 20 o 12 , Molecular weight: 464.38, the structural formula of described hyperoside is as follows:
[0033]
[0034] Take 1,000g of the leaves of Cyperus japonica, add 12 times the amount of 70% ethanol for reflux extraction for 2 hours, filter, add 10 times the amount of 70% ethanol for reflux extraction for 1.5 hours, filter, combine the two extracts, recover the ethanol, and pass the aqueous solution through the The treated D101 macroporous resin column was eluted with water, 10% ethanol, 45% ethanol, and 1% sodium hydroxide solution respectively, and the eluted part of 45% ethanol was collected, concentrated under reduced pressure, and dried to obtain more than 30% rhodium, The mixed total g...
Embodiment 2
[0037]Take 100000g of Huajuhong, add 12 times the amount of 70% ethanol for reflux extraction for 2 hours, filter, add 10 times the amount of 70% ethanol for reflux extraction for 1.5 hours, filter, combine the two extracts, recover ethanol, and pass the aqueous solution through the treated A good D101 macroporous resin column was eluted with water, 10% ethanol, 45% ethanol, and 1% sodium hydroxide solution respectively, and the eluted part of 45% ethanol was collected, concentrated under reduced pressure, and dried to obtain more than 30% rhodium The mixed total glycosides were separated by silica gel column chromatography and Sephadex LH-20 column chromatography, and the rhoradiside fractions were combined and crystallized to obtain the pure rhodoside (450g, purity: 98.9%). UV, IR, ESI-MS, 1 H-NMR, 13 Compared with C-NMR, the structure of Rhodorin was determined.
Embodiment 3
[0039] Take 100000g of Huajuhong, add 10 times the amount of 60% ethanol for reflux extraction for 2 hours, filter, add 8 times the amount of 60% ethanol for reflux extraction for 1.5 hours, filter, combine the two extracts, recover ethanol, and pass through the treated A good D101 macroporous resin column was eluted with water, 10% ethanol, 45% ethanol, and 1% sodium hydroxide solution respectively, and the eluted part of 45% ethanol was collected, concentrated under reduced pressure, and dried to obtain more than 30% rhodium The mixed total glycosides were separated by silica gel column chromatography and Sephadex LH-20 column chromatography, and the rhoradiside fractions were combined and crystallized to obtain the pure rhodoside (455g, purity: 98.6%). UV, IR, ESI-MS, 1 H-NMR, 13 Compared with C-NMR, the structure of Rhodorin was determined.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com