Polyethylene glycol 12-hydroxy stearate-containing danshen root medicament injection preparation and preparation method thereof
A polyethylene glycol lauryl hydroxystearate and injection preparation technology, which is applied in the field of medicine, can solve the problems of poor water solubility of tanshinone and cannot meet the actual clinical needs, and achieve the effect of improving clarity, facilitating clinical medication and popularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
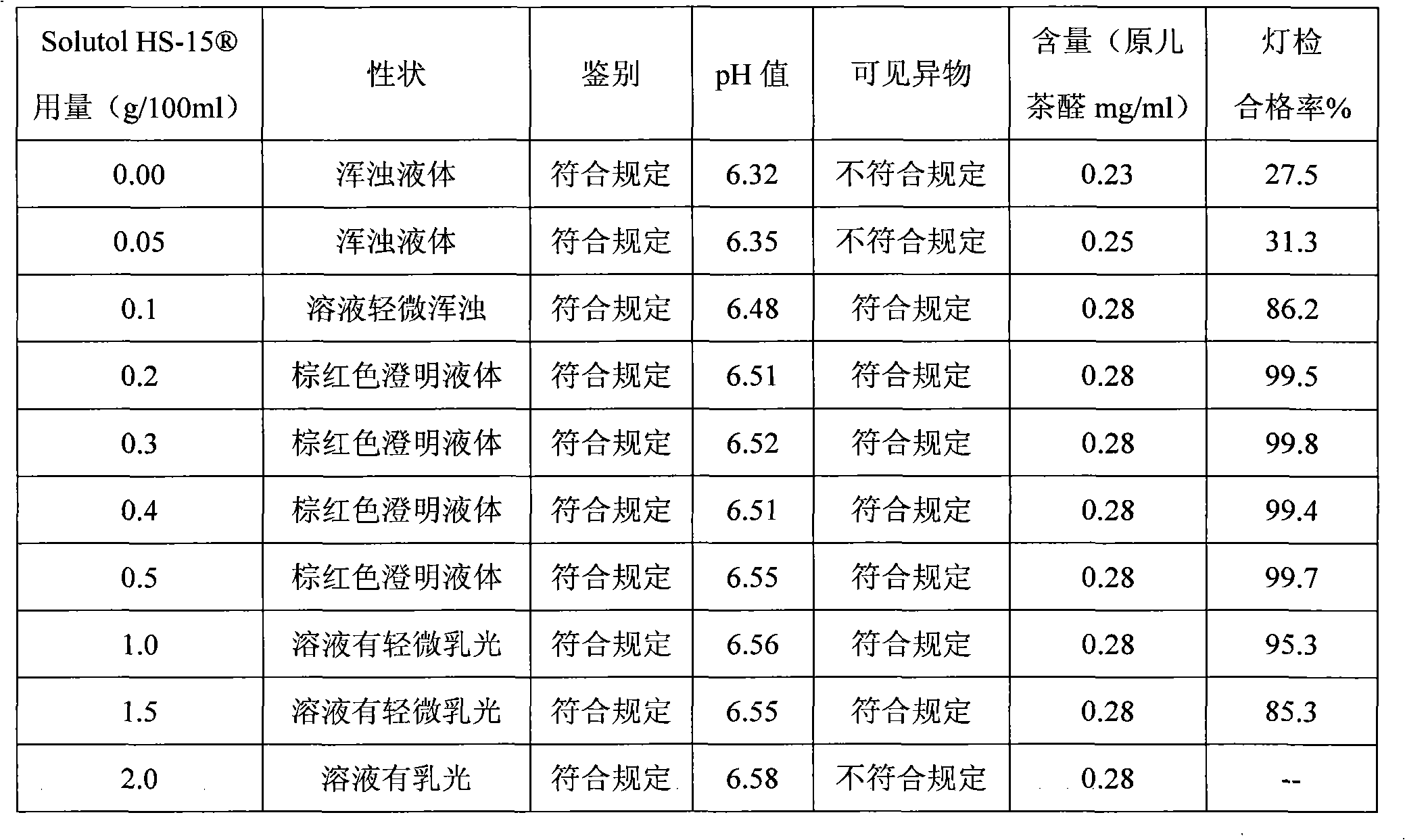
[0027] A salvia miltiorrhiza drug injection preparation containing polyethylene glycol lauryl hydroxystearate, which is mainly prepared by dissolving salvia miltiorrhiza extract and polyethylene glycol lauryl hydroxystearate for improving the clarity of the injection in water for injection The finished medicine for injection, the dosage of the polyethylene glycol lauryl hydroxystearate is 0.1g~1.0g / 100ml.
[0028] The specific preparation method of this embodiment is as follows:
[0029] Danshen 1500g
[0030] Solutol HS- 2.0g
[0031] The dandelions were decocted in water three times, the first time was 2 hours, the second and third time were 1.5 hours respectively, the decoctions were combined, filtered, and the filtrate was concentrated under reduced pressure to 750ml. Add ethanol for precipitation twice, the first time the alcohol content is 75%, the second time the alcohol content is 85%, each time it is refrigerated and then filtered. The filtrate recovers ethanol an...
Embodiment 2
[0033] Danshen 1000-2000g
[0034] Solutol HS- 1.0-10.0g
[0035] The dandelions were decocted in water three times, the first time was 2 hours, the second and third time were 1.5 hours respectively, the decoctions were combined, filtered, and the filtrate was concentrated under reduced pressure to 750ml. Add ethanol for precipitation twice, the first time the alcohol content is 75%, the second time the alcohol content is 85%, each time it is refrigerated and then filtered. The filtrate recovers ethanol and concentrates it to about 250ml. Add water for injection to 400ml, mix well, refrigerate, filter, adjust the pH value to 6.8 with 10% sodium hydroxide solution, boil for half an hour, filter, add polyethylene glycol lauryl hydroxystearate to the filtrate, and add water for injection Prepare a solution with a certain concentration, stir evenly, adjust the pH value to 6.8 with 10% sodium hydroxide solution, filter, subpackage, and freeze-dry to make a freeze-dried powder. ...
Embodiment 3
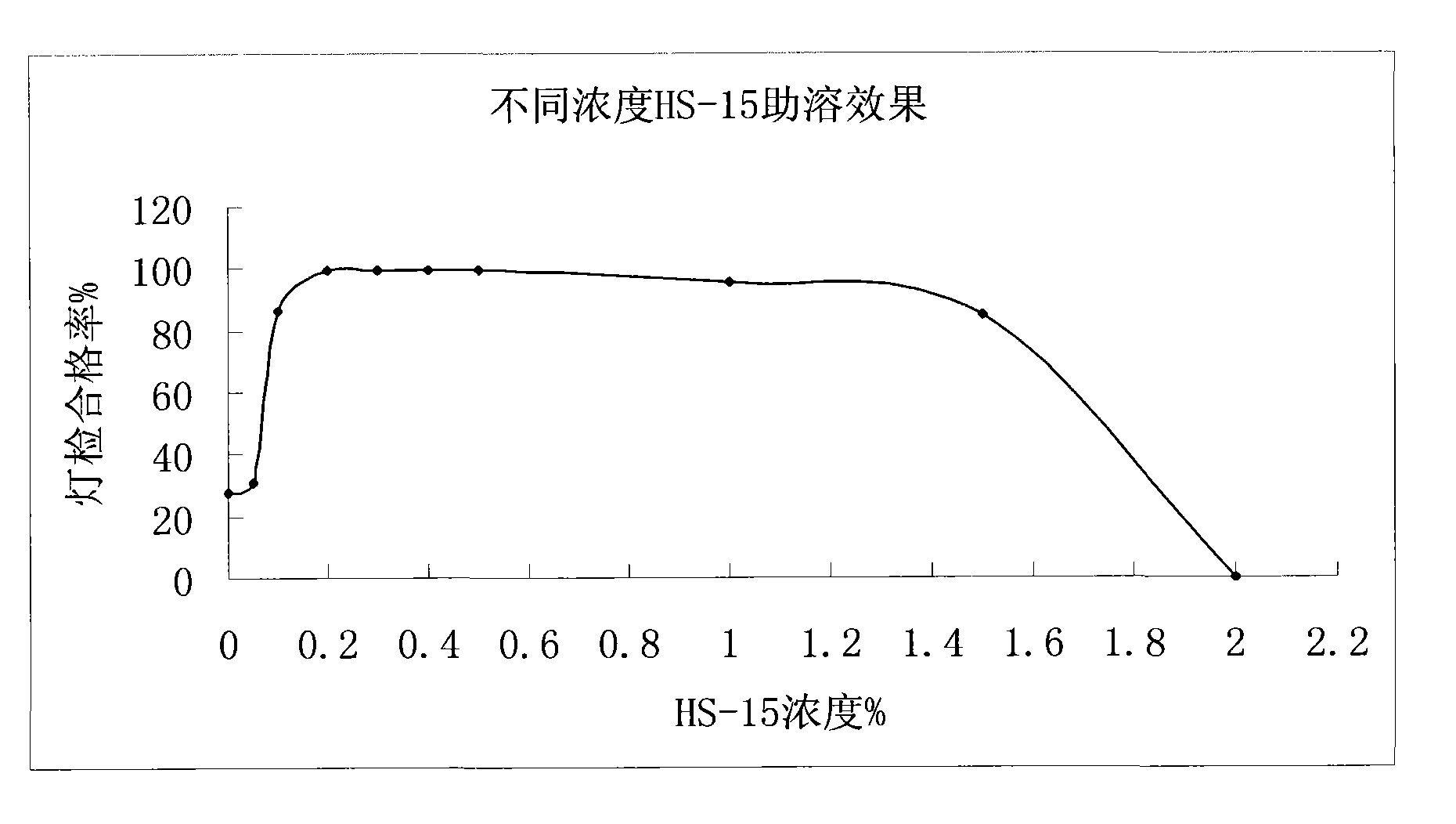
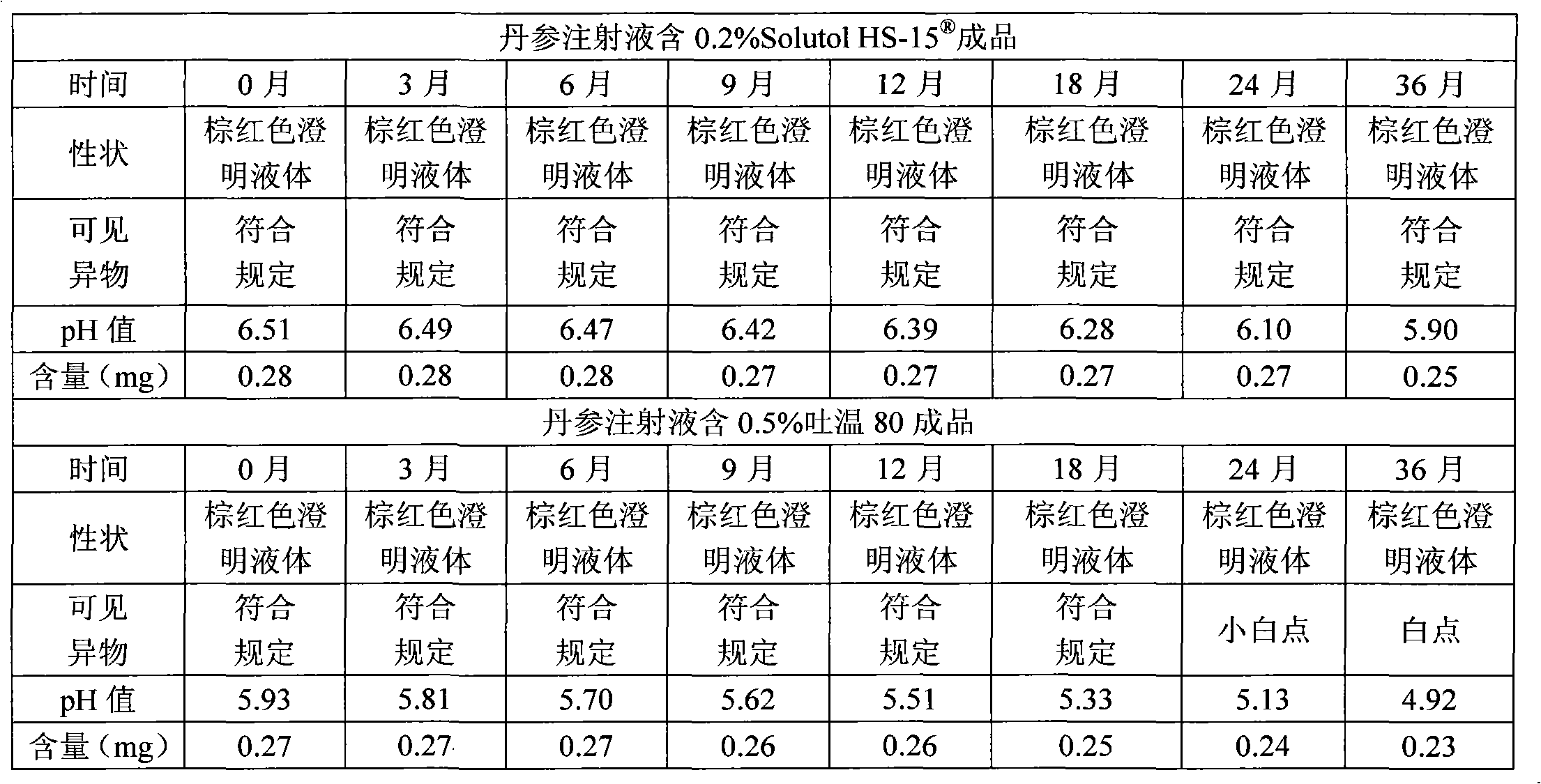
[0037] Polysorbate 80 with Solutol HS- Comparative Test of Stability of Solubilized Danshen Injection
[0038] The solution stability of the salvia miltiorrhiza injection prepared by the invention is very good, which solves the problems that the salvia miltiorrhiza injection is prone to precipitation and solution turbidity during storage and high-temperature sterilization. Utilize the prepared Danshen injection of the present invention according to the relevant requirements of the Chinese Pharmacopoeia 2005 edition two appendices XI X C drug preparation stability test guiding principle, investigated respectively placed at 25 ℃ for 36 months, 40 ℃ for 6 months, 60 ℃ for 20 days of drug stability, the result is that the product quality is stable under the above-mentioned test conditions, and each test index meets the requirements of the quality standard of this product.
[0039] The results of pharmacological experiments show that the Danshen injection prepared by the present ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com