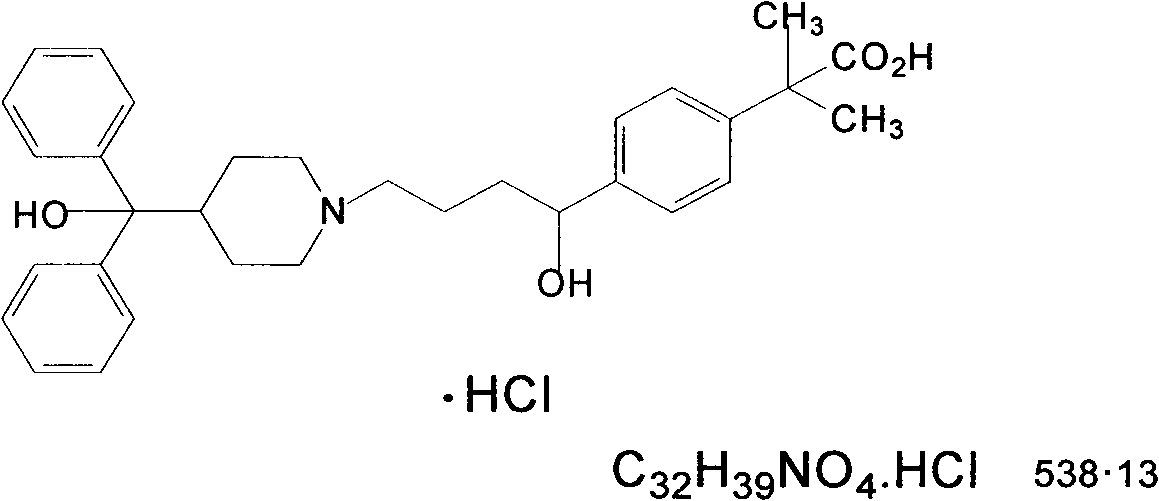
Preparation method for fexofenadine hydrochloride-containing medicinal composition
A technology of fexofenadine hydrochloride and fexofenadine, which is applied in the direction of drug combinations, active ingredients of heterocyclic compounds, sugar-coated pills, etc., can solve the problem of relatively low utilization rate of equipment, inconvenience for patients to carry and use, inactive Problems such as large amount of ingredients are used to achieve the effect of high relative equipment utilization, good response, and reduced energy consumption and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] The preparation of embodiment one 30mg / fexofenadine hydrochloride tablet
[0111] Get 300 grams of fexofenadine hydrochloride, 480 grams of lactose, 300 grams of starch and 60 grams of hydroxypropyl cellulose (the materials are all passed through a 100 mesh sieve) and put in the fast stirring granulator, after fast stirring and mixing for 10 minutes, use 80% ~85% ethanol (v / v) into polyvinylpyrrolidone (k30) 5% (g / l) solution 276 grams. Quickly stir and mix for two minutes, take it out, use a 14-mesh nylon screen to make granules, then dry at 60-65°C, add 60 grams of crospovidone, 8 grams of magnesium stearate, and granulate with a 14-mesh stainless steel mesh. well mixed. After the detection of the intermediate, it is compressed into tablets, coated with 8% Opadry ethanol suspension prepared with 80% to 85% ethanol (v / v), and obtained after packaging.
[0112] The finished product can meet the requirements of the State Food and Drug Administration standard YBH02552...
Embodiment 2
[0113] The preparation of embodiment two 60mg / fexofenadine hydrochloride tablet
[0114] Get 600 grams of fexofenadine hydrochloride, 720 grams of lactose, 480 grams of starch and 80 grams of hydroxypropyl cellulose (materials are all passed through a 100 mesh sieve) and put in the fast stirring granulator, after fast stirring and mixing for 10 minutes, use 80% ~85% ethanol (v / v) into polyvinylpyrrolidone (k30) 5% (g / l) solution 356 grams. Quickly stir and mix for two minutes, take it out, use a 14-mesh nylon screen to make granules, then dry at 60-65°C, add 80 grams of crospovidone, 16 grams of magnesium stearate, and granulate with a 14-mesh stainless steel mesh. well mixed. After the detection of the intermediate, it is compressed into tablets, coated with 8% Opadry ethanol suspension prepared with 80% to 85% ethanol (v / v), and obtained after packaging.
[0115] The finished product can meet the requirements of the State Food and Drug Administration standard YBH02552006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com