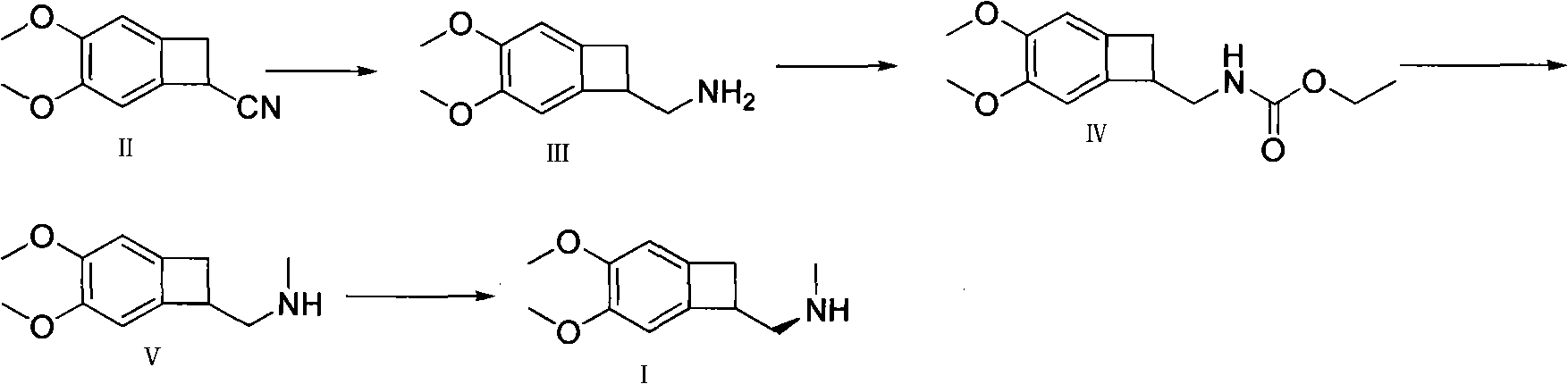
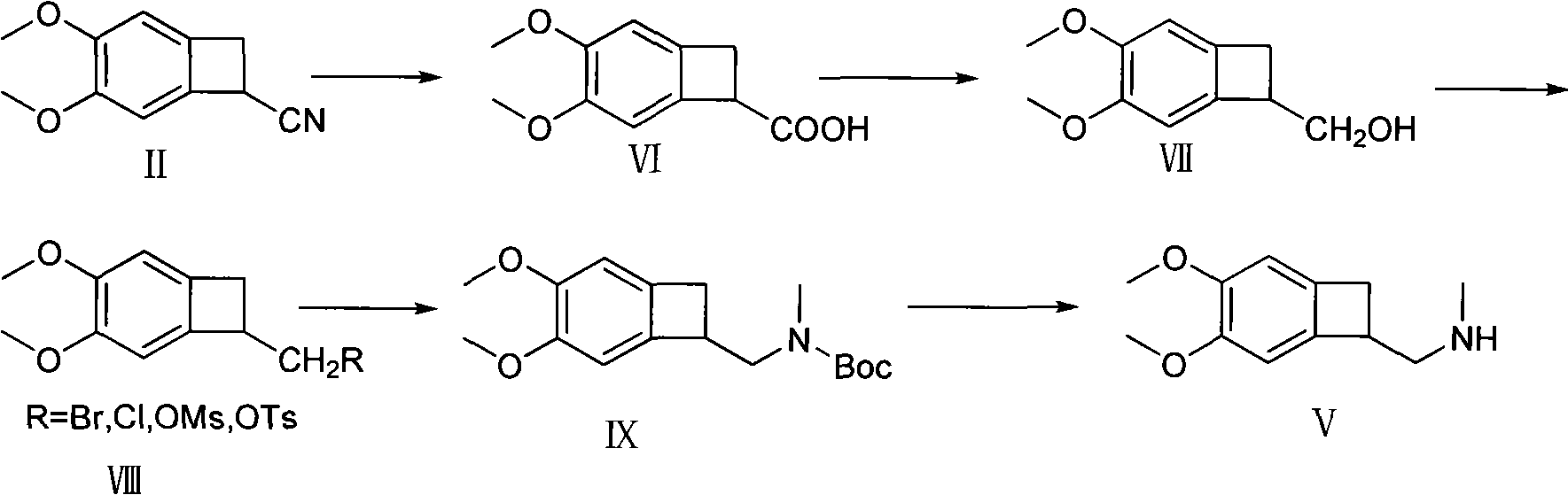
Synthetic method of (1S)-4,5-Dimethoxy-1-(aminomethyl)benzocyclobutane
A methylaminomethyl, benzocyclobutane technology, applied in the preparation of aminohydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problem of low total reaction yield, high production cost, and unsuitable industrialization. production and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035] Example 1 4, the preparation of 5-dimethoxybenzocyclobutane-1-carboxylic acid
[0036] 120 g of 1-cyano-4,5-dimethoxybenzocyclobutane, 300 ml of methanol, and 300 ml of water were added to a 1000 ml reaction flask, and 36 g of sodium hydroxide was added with stirring. Raise the temperature to 80°C and react for 4 hours, TCL detects that the reaction is complete, drop the temperature at 20-25°C and add about 150ml of hydrochloric acid to pH<1, stir and crystallize for two hours, filter, dry at 50-55°C for 8 hours, dry weight 127g, mp: 142.5-143°C, yield 93%.
example 2
[0037] Example 2 Preparation of 4,5-dimethoxybenzocyclobutyl-1-methanol
[0038] Add 31.2g of 4,5-dimethoxybenzocyclobutane-1-carboxylic acid and 300ml of tetrahydrofuran to a dry 1000ml reaction flask, lower the temperature, add 21g of sodium borohydride at t<0°C, and dropwise add 25.8g of sulfuric acid , keep warm for 10nm, stir naturally for 1 hour, reflux for 30min, drop the temperature at t<0°C and add 180ml of methanol dropwise. After the dropwise addition, reflux for 30nm, evaporate to dryness under reduced pressure, add 200ml of dichloromethane and 500ml of water, separate layers, extract the water layer with 100ml of dichloromethane, wash the organic layer with 300ml of water, combine the organic layers, and reduce the pressure below 50°C The organic layer was evaporated to dryness to obtain 29.4 g of an oily substance.
example 3
[0039] Example 3 Preparation of 4,5-dimethoxybenzocyclobutyl-1-methylsulfonate
[0040] Add 29.4g of 4,5-dimethoxybenzocyclobutyl-1-methanol to 100ml of dichloromethane to dissolve, add 10ml of pyridine and dropwise add 25.4g of methanesulfonyl chloride at t<0°C, after the addition is completed, 8-12 Incubate at ℃ for 6-8 hours, TCL detection (chloroform:methanol=3:1), t<20 ℃, add 10% sulfuric acid to wash the organic layer to PH<2, let stand to separate the layers, extract the water layer with 100ml dichloromethane, organic The layers were washed with 300 ml of water, and the organic layers were combined. The organic layer was evaporated to dryness under reduced pressure below 50°C, and 100ml of isopropanol was added to crystallize. Filter, dry at 50-55°C for 8 hours, dry weight 36.7g, mp: 97-98°C, yield 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com