Novel penetration and controlled-release medicament delivery system and preparation method thereof
A technology of delivery system and controlled release drug, which is applied in the directions of pharmaceutical formulations, medical preparations with non-active ingredients, and pill delivery.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
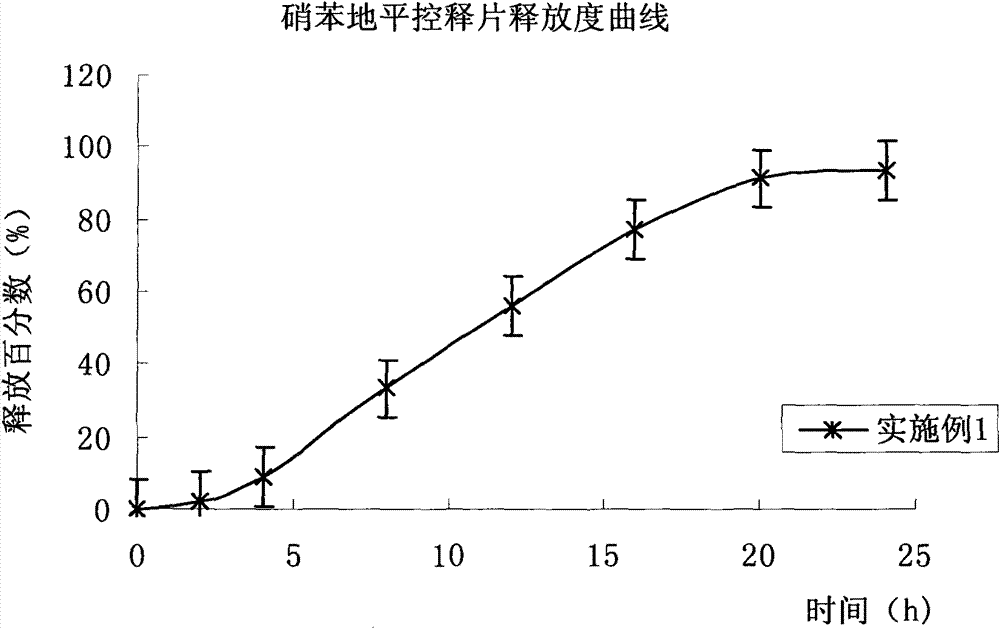
Embodiment 1
[0078] prescription:
[0079] (1) Drug-containing layer (per tablet)
[0080]
[0081]
[0082] (2) booster layer (per piece)
[0083]
[0084] (3) Composition of isolation coat coating solution
[0085]
[0086] (4) Composition of semipermeable membrane (controlled release coating) coating solution
[0087]
[0088] (5) The composition of the coating solution for the beautiful coat:
[0089] Chambray Pink 16g
[0090] water 90ml
[0091] 16mg (5%) weight gain per tablet
[0092] Preparation Process
[0093] (1) Preparation of drug-containing layer particles
[0094]Avoid light operation. Mix the prescribed amount of nifedipine passing through a 60-mesh sieve and auxiliary materials evenly, add it into the fluidized bed, spray 95% ethanol-water solution to granulate, and stop granulation when it reaches a suitable particle size, carry out drying, and pass through a 20-mesh sieve , whole grain, add magnesium stearate, mix well, set aside;
[0095] (2) P...
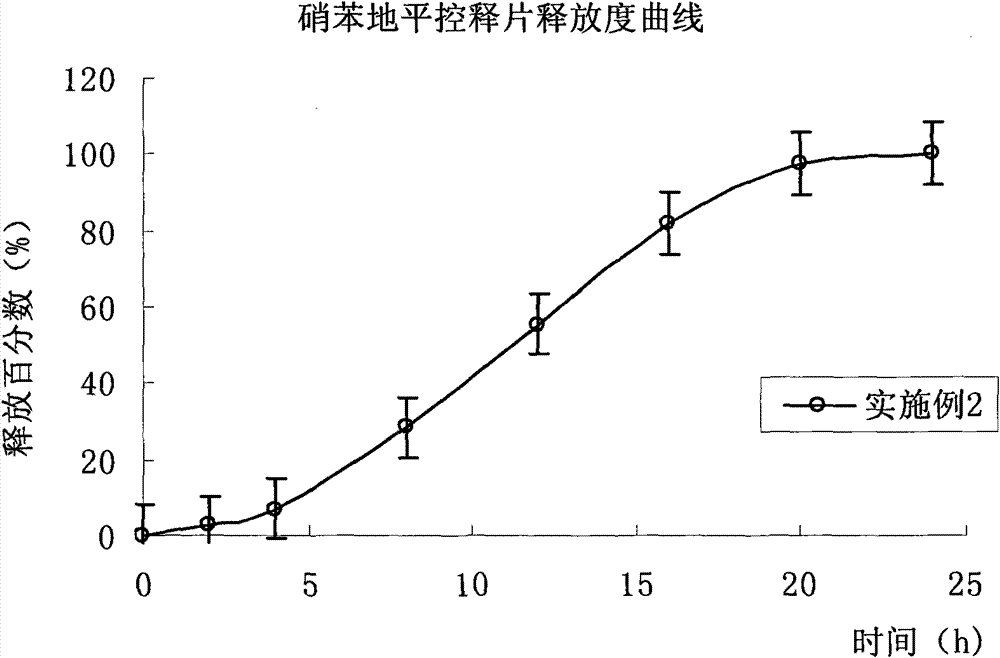
Embodiment 2
[0111] prescription:
[0112] (1) Drug-containing layer (per tablet)
[0113]
[0114] (2) booster layer (per piece)
[0115]
[0116] (3) Composition of isolation coat coating solution
[0117]
[0118]
[0119] (4) Composition of semipermeable membrane (controlled release coating) coating solution
[0120]
[0121] (5) The composition of the coating solution for the beautiful coat:
[0122] Chambray Pink 16g
[0123] water 90ml
[0124] 16mg (5%) weight gain per tablet
[0125] The preparation process and drug release determination method are the same as in Example 1.
[0126] Drug release results: The average cumulative drug release percentages at 2, 4, 8, 12, 16, 20 and 24 hours were 2.5%, 7.1%, 28.3%, 55.5%, 81.8%, 97.6% and 100.2%, respectively. (Release curve see figure 2 ).
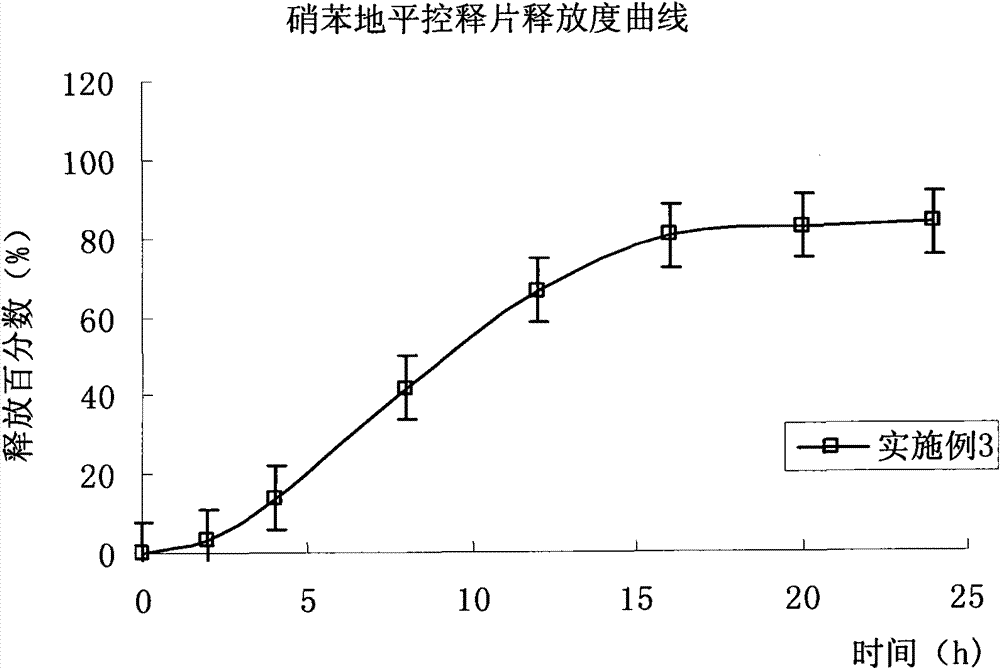
Embodiment 3
[0128] Except not adding isolation gown, prescription and preparation process are the same as embodiment 2.
[0129] Drug release results: the average cumulative drug release percentages at 2, 4, 8, 12, 16, 20 and 24 hours were 3.2%, 13.8%, 41.8%, 66.3%, 80.2%, 82.5% and 83.4%, respectively. (Release curve see image 3 ).
[0130] Compared with Example 2, the results of drug release in this example show that the drug release is faster in the early stage and the final cumulative release amount of the drug is relatively small under the condition of no isolation gown. Nifedipine has good solubility in acetone, and the adhesion of the drug on the controlled-release coating leads to a low final release of the drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com