Method for analyzing contents and valence states of metals inside and outside doped mesoporous molecular sieve framework
A mesoporous molecular sieve and molecular sieve technology are applied in the field of analyzing the content and valence of metal elements inside and outside the doped mesoporous molecular sieve framework, which can solve the problem of inaccuracy and analyze the content and valence of doped metal ions inside and outside the molecular sieve framework. description and other problems, to achieve the effect of a simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
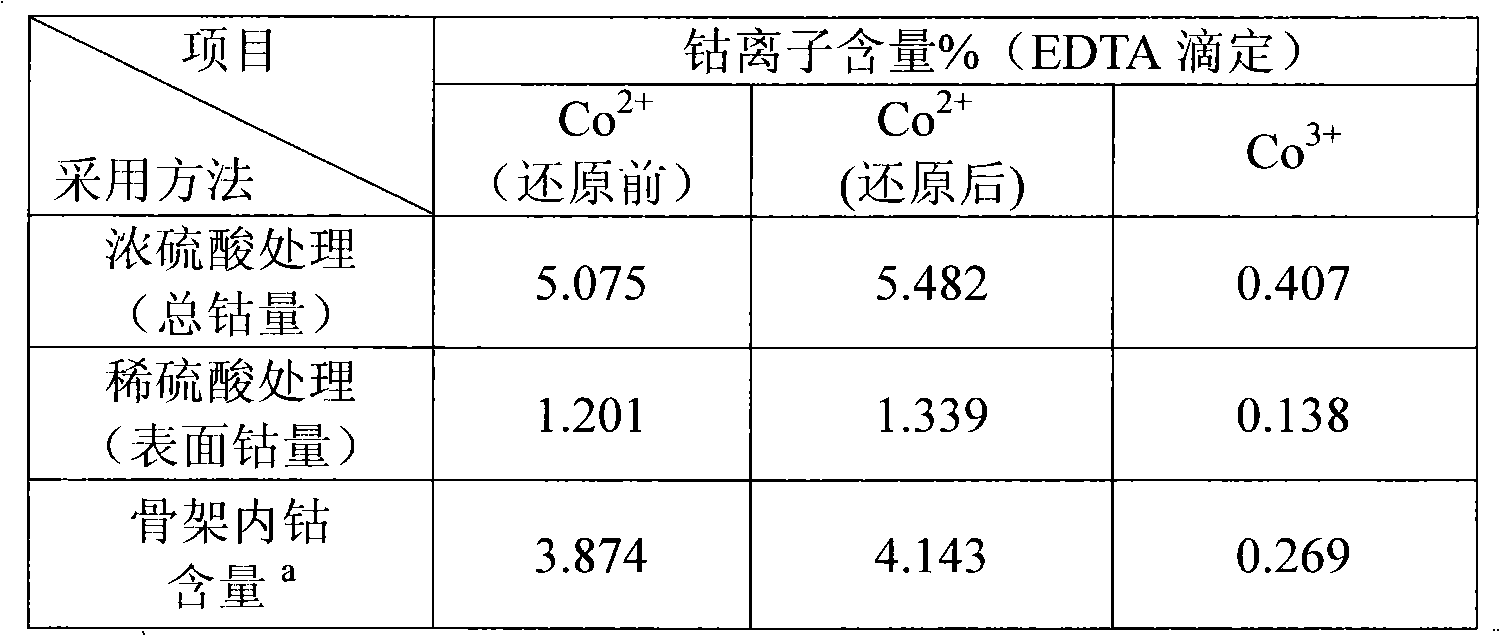
[0022] Example 1: Analysis of MCM-41 containing cobalt. Take 0.1g of the sample and soak in 2mL of 18mol / L concentrated sulfuric acid at 100°C for 12h, and dissolve and stir 20mL of 0.5mol / L dilute sulfuric acid at 15°C for 10min. The mixture is filtered with deionized water by centrifugation. No metal ions can be detected in the residue. The metal ion clear liquid is obtained and the volume is fixed with a volumetric flask. The obtained clear liquid is fixed to volume with malachite green as a contrast agent and xylenol orange as an indicator. = 5-6 hexamethylenetetraammonium buffer system uses EDTA to directly titrate the divalent cobalt ion, and the trivalent cobalt ion can be reduced by KI and then titrated with EDTA.
[0023] The specific results are shown in Table 1. The data is the mass percentage of cobalt in the material.
[0024] Table 1
[0025]
[0026] a: Total cobalt amount-surface cobalt amount.
Embodiment 2
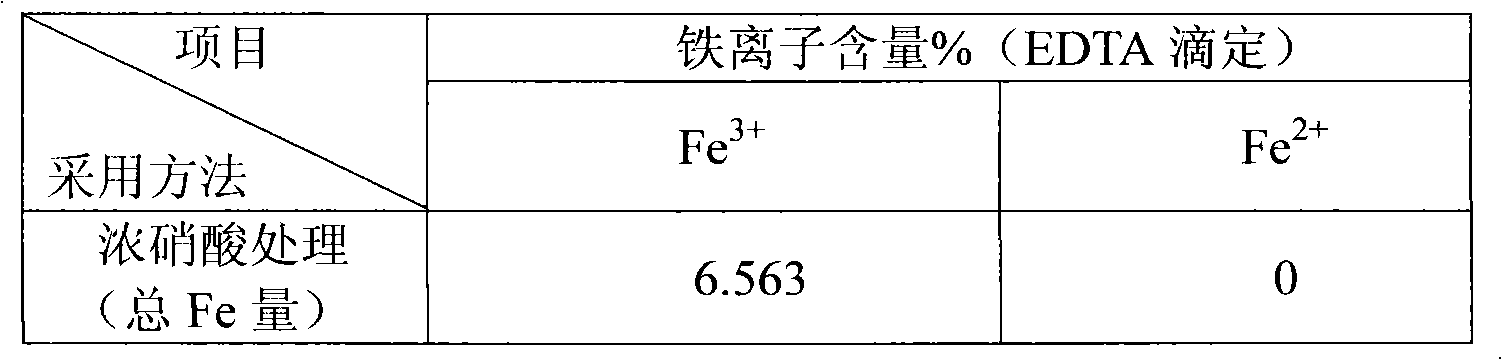
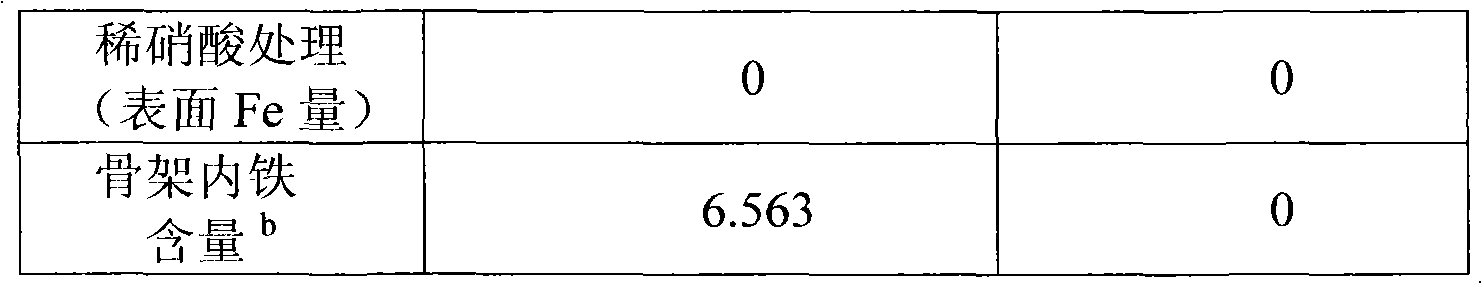
[0027] Example 2: Analysis of iron-containing MCM-41. Take 0.1g of the sample and soak 4mL of 16mol / L concentrated nitric acid at 100℃ for 12h, and dissolve and stir 20mL of 0.5mol / L dilute nitric acid at 20℃ for 10min. The mixture was filtered with deionized water by centrifugation, and no metal ions were detected in the residue. The resulting clear liquid was constant volume, using sulfosalicylic acid as an indicator, and hydrochloric acid to control the pH=1.5-2 when the ferric ion was directly titrated with EDTA. Since the synthesized sample uses ferric nitrate as the iron source and is calcined at 550°C, there is no ferrous ion in the sample. The specific results are shown in Table 2. The data is the mass percentage of iron in the material.
[0028] Table 2
[0029]
[0030]
[0031] b: Total iron content-surface iron content.
example 3
[0032] Example 3: Analysis of vanadium-containing MCM-41. Take 0.1g of the sample and soak it with 5mL of 6mol / L sodium hydroxide at 60℃ for 6h, and dissolve 40mL of 0.5mol / L sodium hydroxide at 20℃ for 10min. The mixture was filtered with deionized water by centrifugation. No metal ions could be detected in the residue. The resulting clear liquid was fixed to volume. Using N-phenyl-anthranilic acid as an indicator, the pentavalent vanadium was directly titrated with ferrous ammonium sulfate under strong acid conditions. The tetravalent vanadium can be oxidized to pentavalent by potassium permanganate and analyzed. The specific results are shown in Table 3. The data is the mass percentage of vanadium in the material.
[0033] table 3
[0034]
[0035] c: Total vanadium content-surface vanadium content.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap