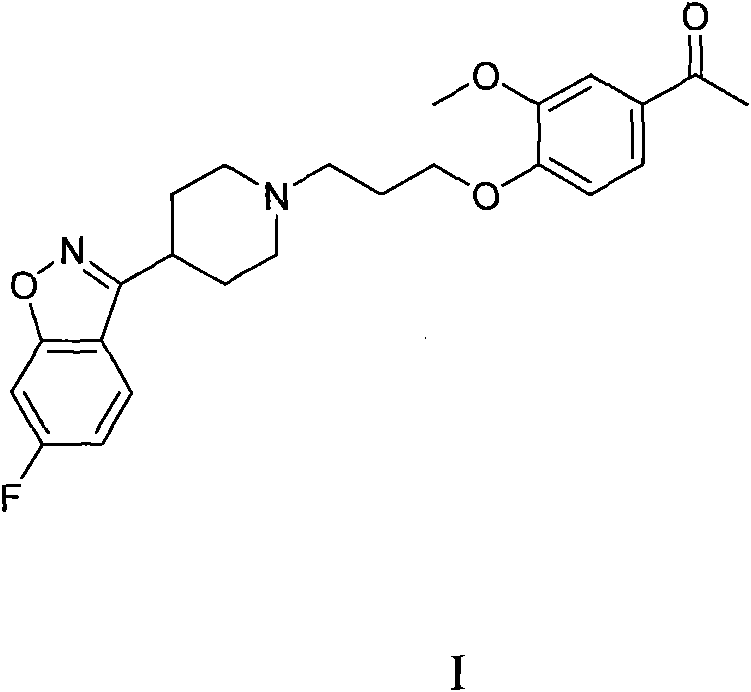
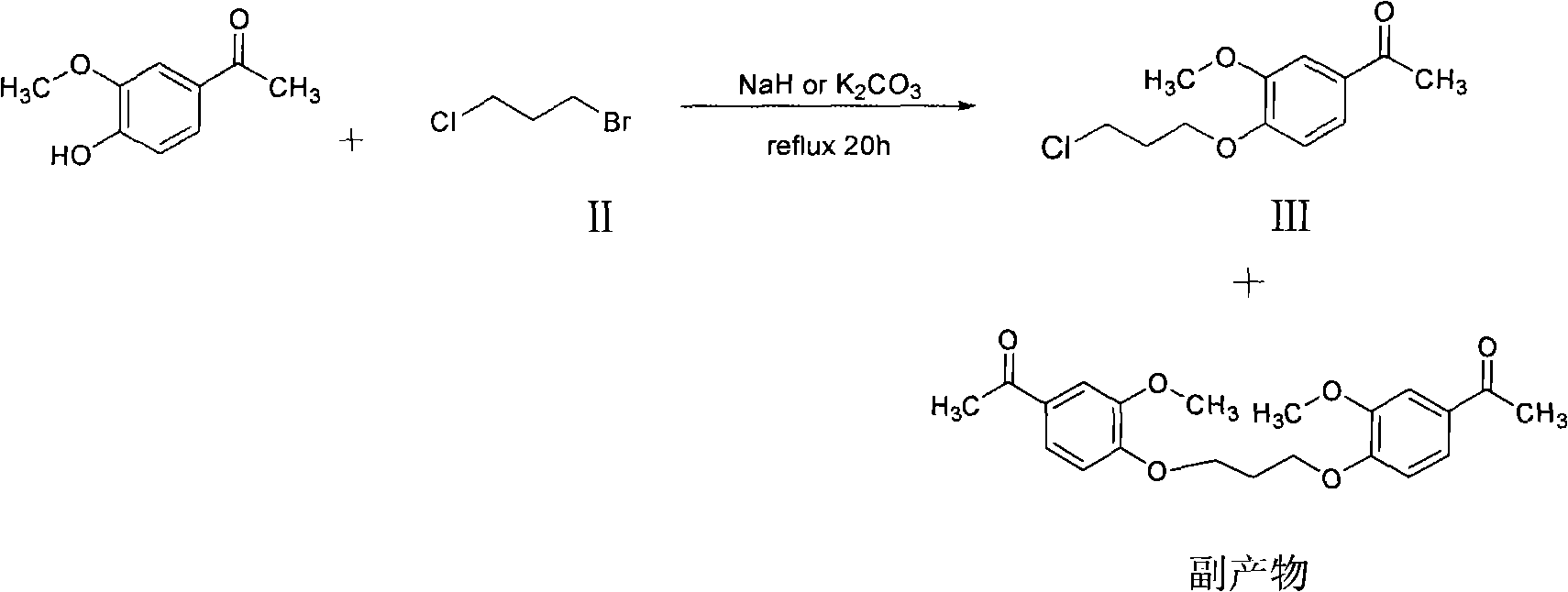
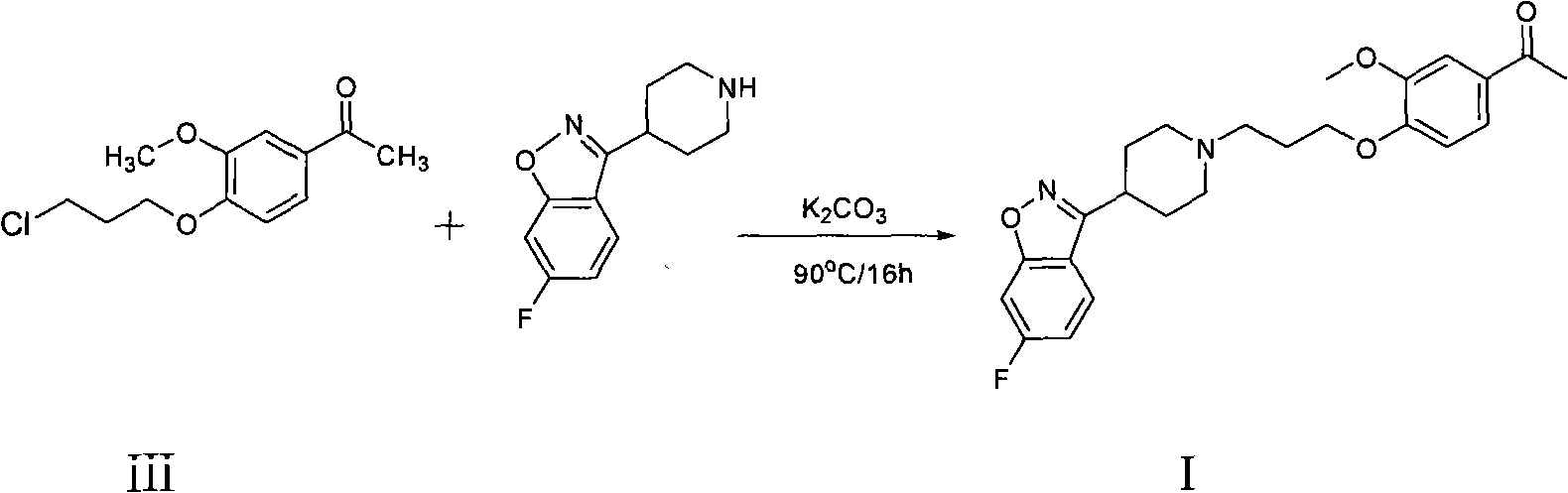
Preparation method of Iloperidone
A technology of ethyl ketone and methoxyacetophenone, applied in nervous system diseases, organic chemistry, drug combination, etc., can solve problems such as long reaction time, increase reaction cost, increase operation difficulty, etc., and shorten reaction time , Prevention of by-products, strong controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 4-Hydroxy-3-methoxyacetophenone (88.0g, 0.53mole), K 2 CO 3 (69.1g, 0.5mol) was added to 375ml of acetone solution and heated to reflux. After refluxing for 30 minutes, a mixed solution of 1-chloropropanol (66.1 g, 0.70 mol) and acetone (75 ml) was added dropwise to the reaction solution. Stir evenly, and keep the reaction at 50°C for 5 hours. After the reaction, the solid was filtered out, the filtrate was concentrated, and the remaining oily liquid was slurried with petroleum ether to obtain 110 g (93%) of an off-white solid, which was pure 1-[4-(3-hydroxypropoxy)-3-methyl Oxyphenyl] ethyl ketone m.p. 161-163°C.
Embodiment 2
[0022] A mixture of p-toluenesulfonyl chloride (3.8g, 0.02mol) and dimethylformamide (10ml) was added dropwise to 1-[4-(3-hydroxypropoxy)-3-methoxyphenyl]ethyl In a mixed system composed of ketone (5.3g, 0.022mol) and dimethylformamide (60ml), stir at room temperature for 30 minutes, add 6-fluoro-3-(4-piperidinyl)-1,2-benzo Isoxazole hydrochloride (5.1g, 0.02mol), K 2 CO 3 (5.2g, 0.04mol), and react at 90°C for 3 hours. The mixture was poured into water, extracted with ethyl acetate, the ethyl acetate layer was washed with water, MgSO 4 Dry and concentrate to give a brown solid. Recrystallized with ethanol to obtain 7.0 g (82%) of 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propane Oxy]-3-methoxyphenyl]ethyl ketone as a beige solid, m.p. 118-120°C.
[0023] NMR analysis:
[0024] 1 H NMR (400MHz, DMSO-d 6 )δ6.85-7.20 (m, 3H), 3.90-4.02 (t, 2H), 3.70-3.75 (s, 2H), 3.48-3.59 (t, 2H), 2.51-2.63 (s, 3H), 2.03- 1.99(s, 1H), 1.82-1.91(m, 2H).
[0025] 13 C N...
Embodiment 3
[0030] A mixture of p-toluenesulfonyl chloride (3.8g, 0.02mol) and toluene (10ml) was added dropwise to 1-[4-(3-hydroxypropoxy)-3-methoxyphenyl]ethylketone (5.3 g, 0.022mol) and toluene (60ml) in a mixed system composed of toluene (60ml), stirred at room temperature for 30 minutes, added 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride (5.1 g, 0.02mol), K 2 CO 3 (5.2g, 0.04mol), heated and stirred at 90°C for 3 hours. The mixture was poured into water, extracted with ethyl acetate, the ethyl acetate layer was washed with water, MgSO 4 Dry and concentrate to give a brown solid. Recrystallized from ethanol to obtain 6.8 g (80%) of 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propane Oxy]-3-methoxyphenyl]ethyl ketone as a beige solid, m.p 118-120°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com