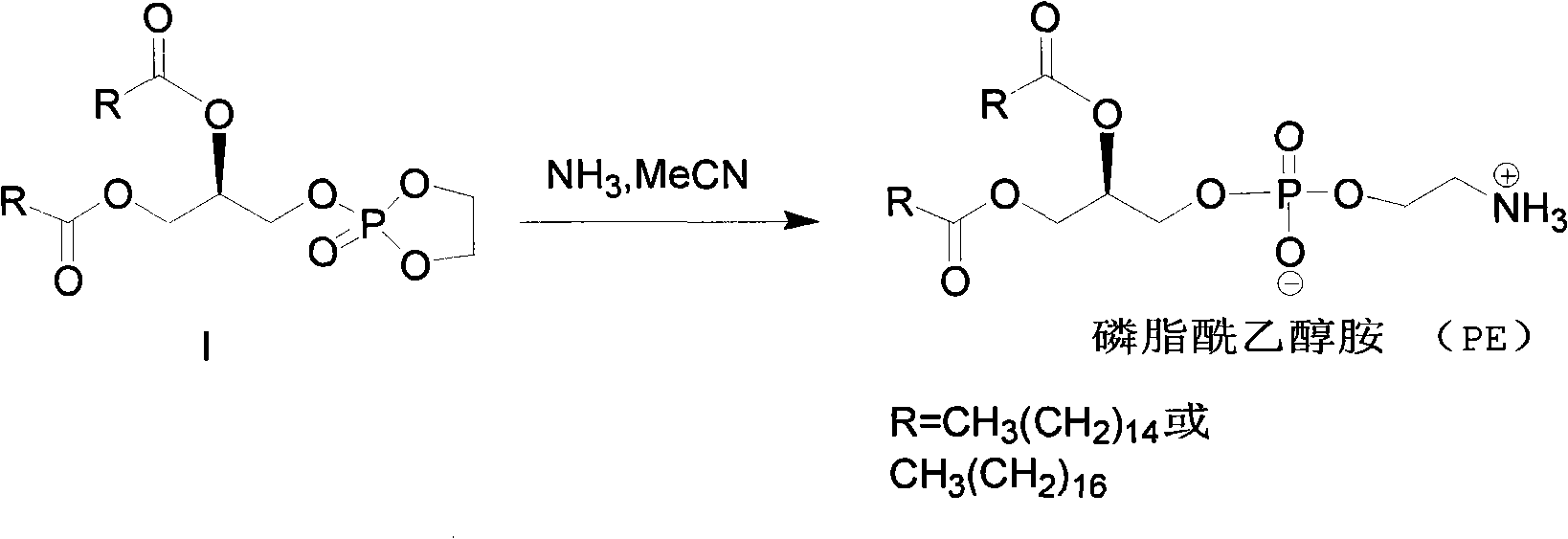
Method for synthesizing PE (Phosphatidyl Ethanolamine)
A technology for synthesizing phosphatidylethanolamine and phosphatidylethanolamine, which is applied in the direction of phosphorus organic compounds, can solve the problems of low yield, large by-products, and unsuitability for industrial production, and achieve the effects of cost reduction, simple operation, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
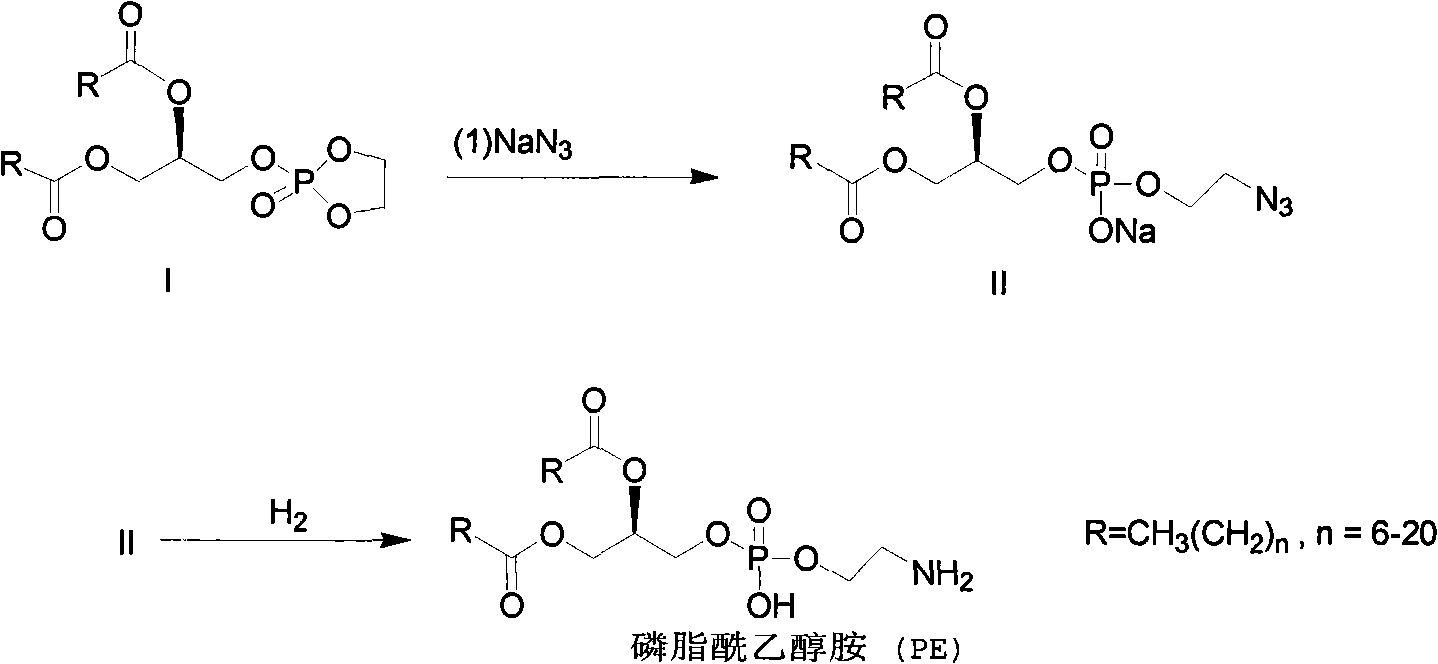
[0020] 1.1 Compound II [R=CH 3 (CH 2 ) 14 ] preparation
[0021] Add 1.20g of compound I, 15ml of DMF and 0.23g of NaN at room temperature 3 , nitrogen protection, oil bath heating reaction, control the oil bath temperature 80 ~ 90 ℃, react for about 16h, after the reaction, cool to room temperature, add 40ml of methanol to dilute, filter, and distill the filtrate under reduced pressure to remove methanol at the water bath temperature below 60 ℃; Under the oil bath, the water pump distilled off DMF under reduced pressure, replaced it with an oil pump, and sucked the remaining DMF under reduced pressure to obtain 1.15 g of solid (compound II), with a yield of 88%.
[0022] 1 HNMR (CDCl 3 , 500 MHz): δ=0.88(t, 6H), 1.25(m, 52H), 2.30(m, 4H), 3.48(s, 2H), 3.99(s, 2H), 4.02(d, 2H), 4.18 (m, 1H), 4.40(d, 1H), 5.25(s, 1H).
[0023] MS (EI): m / e=740.
[0024] 1.2 Preparation of Compound PE, Phosphatidylethanolamine [R=CH 3 (CH 2 ) 14 ]
[0025] Add 1.15g raw material (com...
Embodiment 2
[0029] 2.1 Compound II [R=CH 3 (CH 2 ) 16 ] preparation
[0030] Add 1.29g of compound I, 15ml of DMF and 0.23g of NaN at room temperature 3 , nitrogen protection, oil bath heating reaction, control the oil bath temperature 80 ~ 100 ℃, react for about 16h, after the reaction, cool to room temperature, add 400ml of methanol to dilute, filter, and distill the filtrate to remove methanol under reduced pressure at a water bath temperature below 60 ℃; Under the oil bath, the water pump distilled off DMF under reduced pressure, replaced it with an oil pump, and suctioned the remaining DMF under reduced pressure to obtain 1.24 g of solid (Compound II), with a yield of 88%.
[0031] 1 H NMR (CDCl 3 , 500 MHz): δ=0.88(t, 6H), 1.25(m, 60H), 2.30(m, 4H), 3.48(s, 2H), 3.99(s, 2H), 4.02(d, 2H), 4.18 (m, 1H), 4.40(d, 1H), 5.25(s, 1H).
[0032] MS (EI): m / e=796.
[0033] 2.2 Preparation of compound PE, namely phosphatidylethanolamine [CH 3 (CH 2 ) 16 ]
[0034] Add 1.24g of raw m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com