Method for purifying active ingredients of compound Naodesheng
A technology of active ingredients and purification methods, applied in the field of purification of active ingredients of compound Naodesheng, can solve the problems that affect the research and development of new traditional Chinese medicine compound drugs and the process of internationalization, are not conducive to the development of traditional Chinese medicine, and are inconsistent with the modernization of traditional Chinese medicine, etc. To achieve the effect of stable and uniform dry powder, shorten heating time and reduce loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The extraction process experiment of the first part of embodiment 1
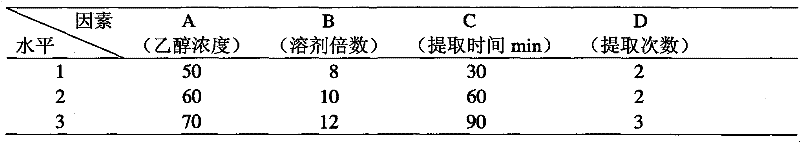
[0031] Experiments were carried out using the ethanol reflux extraction method, using L 9 (3 4 ) Orthogonal experimental method to investigate the process parameters of the first part of ethanol reflux extraction ethanol dosage (multiple of medicinal material quality), ethanol concentration, extraction time, extraction times and other factors, and use ultraviolet-visible spectrophotometry to analyze the total flavonoids and total flavonoids in the extract. The content of saponins was determined, and the contents of puerarin, ferulic acid, ginsenoside Rb1, ginsenoside Rg1 and notoginsenoside R1 were determined by high performance liquid chromatography at the same time, and these were used as indicators for comparative analysis. The results are shown in Table 1 to Table 6.
[0032] Table 1 The first part of the extraction process factor level table
[0033]
[0034] Table 2 Determination results C...
Embodiment 2
[0053] The extraction process experiment of the second part of embodiment 2
[0054] (1) use the water bath temperature immersion extraction method to carry out experiments, adopt L 9 (3 4 ) Orthogonal experiment method to investigate the process parameters of the second part of water bath extraction (the multiple of medicinal material quality), extraction temperature, warm immersion time, leaching times and other factors, and adopt high performance liquid phase method to measure hydroxyl safflower yellow A content, and use this as an indicator for comparative analysis. The results are shown in Tables 7-9.
[0055] Table 7 Safflower extraction process factor level table
[0056]
[0057] Table 8 safflower extraction process orthogonal test results
[0058]
[0059] Table 9 analysis of variance
[0060]
[0061]
[0062] Orthogonal test results show (Table 8, Table 9): the amount of water added, extraction time, and extraction temperature all have a significant...
Embodiment 3
[0066] The resin purification process experiment of the first part of embodiment 3
[0067] The extract obtained by extracting with the aforementioned optimal extraction conditions is carried out as follows:
[0068] (1) Screening of resin
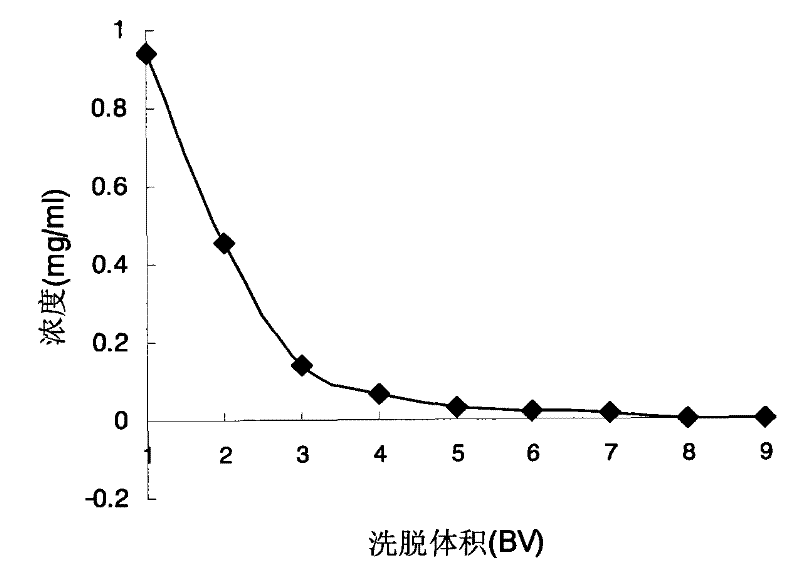
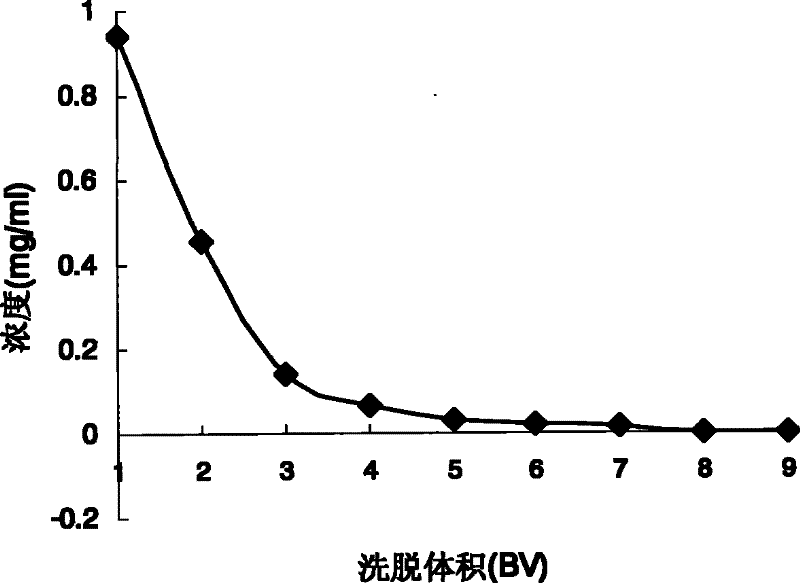
[0069] For the 26 resin columns that have been processed: polyamide resin (including 80-100 mesh, 30-60 mesh, 14-30 mesh), macroporous adsorption resin (including D101, D101B, DM130, HP20, S-8, D4020 , NKA-9, HPD-100, AB-8, XDA-1, XDA-5, H-20, H-60, LSA-40), ion exchange resin (including anion and cation), epoxy resin, polyester Resin, natural resin, ketone-formaldehyde resin, phenolic resin, decolorizing resin, amino resin, etc., accurately absorb 26 parts of the first part of the extract, put it on the column, absorb it overnight, and then elute with distilled water and 70% ethanol in turn, and collect each part of the elution 26 samples were processed completely in parallel (injection speed 1ml / min, elution speed 2ml / min). The index ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com