Arginine hybrid cell-penetrating peptide and application thereof
A technology of arginine and membrane-penetrating peptides, which is applied in the direction of peptides, preparations for in vivo tests, and non-effective ingredients of polymer compounds, and can solve problems such as expensive, weak targeting, and unsuitable for commercial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
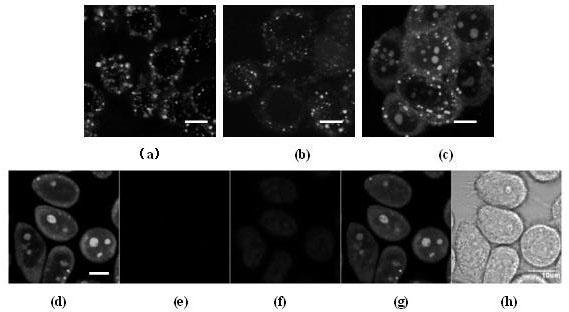
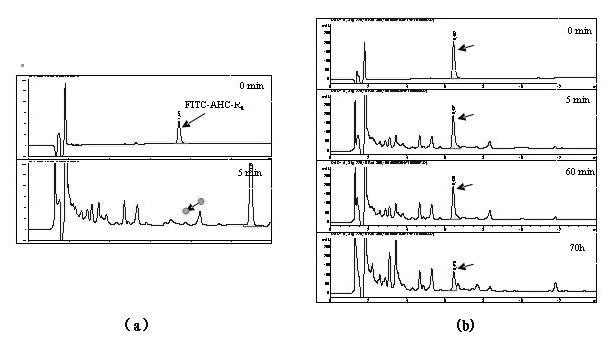
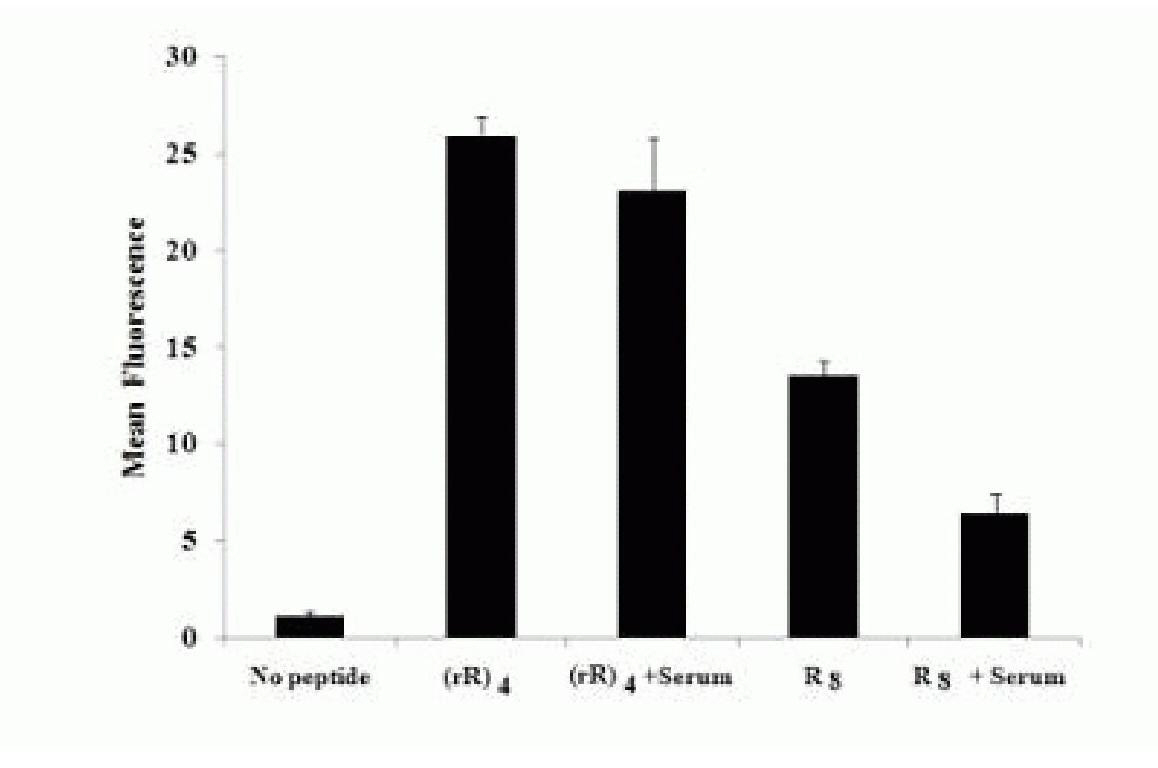
[0026] The non-natural D-arginine (r=D-arginine) and the natural L-arginine (R=L-arginine) are alternately linked into an octameric peptide, named (rR) 4 . As a control group, an all-L-arginine octamer was designed and named as R 8 . R 8 The membrane penetration efficiency is much higher than that of traditional TAT 49-57 protein, but because it also relies on endocytosis, it is trapped in the vesicle after penetrating the membrane. The two membrane-penetrating peptides were labeled with the common non-toxic fluorescein FITC, respectively.
[0027] In the experiments of detecting cell-penetrating peptides, different research groups often have different results in detecting the penetration of membrane-penetrating peptides into cells. The experimental procedures and results of different research groups show that slight changes in experimental conditions will affect the results of cell experiments. Studies have shown that if the method of fixing cells first and then observing...
Embodiment 2
[0047] According to the above steps, we have successively carried out verification tests of arginine cell penetrating peptides with other structures, including (rR) 3 , (rR) 4 r, etc., the various conclusions drawn from these experiments are also consistent with (rR) 4 The situation is basically the same, which shows that it is constructed by hybridization of non-natural D-arginine (r) and natural L-arginine (R), and the structure is (r) n (R) m Arginine cells can carry markers and cargo molecules into the cell, and have the characteristics of high membrane penetration efficiency, strong resistance to enzymatic hydrolysis, specific labeling of living cell nuclei, and low cytotoxicity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com