Bone morphogenetic protein (bmp)-binding domains of proteins of the repulsive guidance molecule (rgm) protein family and functional fragments thereof, and use of same
A morphogenetic protein, binding domain technology, applied in animal/human protein, anti-growth factor immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
[0085] SGSHAPASDDTPE
[0086] SHLNSAVDGFDSE
[0087] LSLRGGGSSGALRGGGGGGRGGGVGSGG
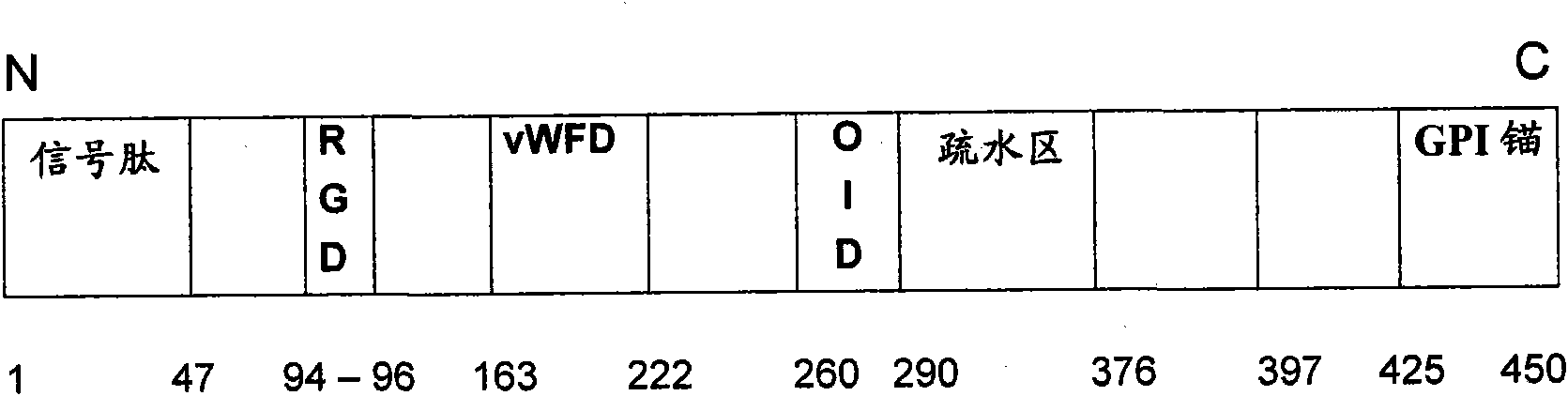
[0088] Examples of BMP-binding domains include amino acid sequences ranging from amino acid 30 to 180 of SEQ ID NO: 2, amino acid 80 to 230 of SEQ ID NO: 4, or amino acid 20 to 150 of SEQ ID NO: 6, or Its functional regenerative protein receptor-binding fragment. These binding domains (and fragments derived therefrom) are also referred to as high-affinity BMP-binding domains. In particular the high-affinity BMP-binding domain also has a high-affinity interaction with the regeneration protein and is therefore also referred to as a high-affinity regeneration protein-binding domain. On the other hand, an example of a low affinity regenerative protein-binding domain is the RGM A fragment 218-284 of SEQ ID NO: 2 described in WO 2007 / 039256 (the disclosure of which is expressly incorporated herein by reference). is not limited thereto, for example when K D (dissociation constant)>1 μM, such as 2...
Embodiment 1
[0636] Production Example 1: Production of RGMA Protein in Mammalian Cells
[0637] To characterize active RGM A, the following RGM A molecules were expressed as Fc fusion proteins in mammalian cells (HEK293):
[0638] -41-168 / Xa
[0639] -47-90
[0640] -47-168
[0641] -316-386
[0642] -1-450
[0643] For this purpose, the DNA encoding the specific molecule was cloned into the vector pcDNA3.1 (+) ZeoIgK / Xa / hIgG lambda hc 257-stop) x HindIII / EcoRI / phosphatase (Invitrogen). To this end, DNA encoding specific fragment regions was amplified by PCR from an RZPD clone (clone AL136826 (DKFZp434D0727); published RZPD sequence: BC015886, AL136826). For this purpose the oligonucleotide primers listed below derived from the published RGMA sequence (published sequence: NM_020211) were used.
[0644] PCR was performed in each case on the RZPD clone pSport-1 DKFZp434D0727 mentioned above using these primers and AccuPrime polymerase. After purifying the PCR product, it was digested...
Embodiment 2
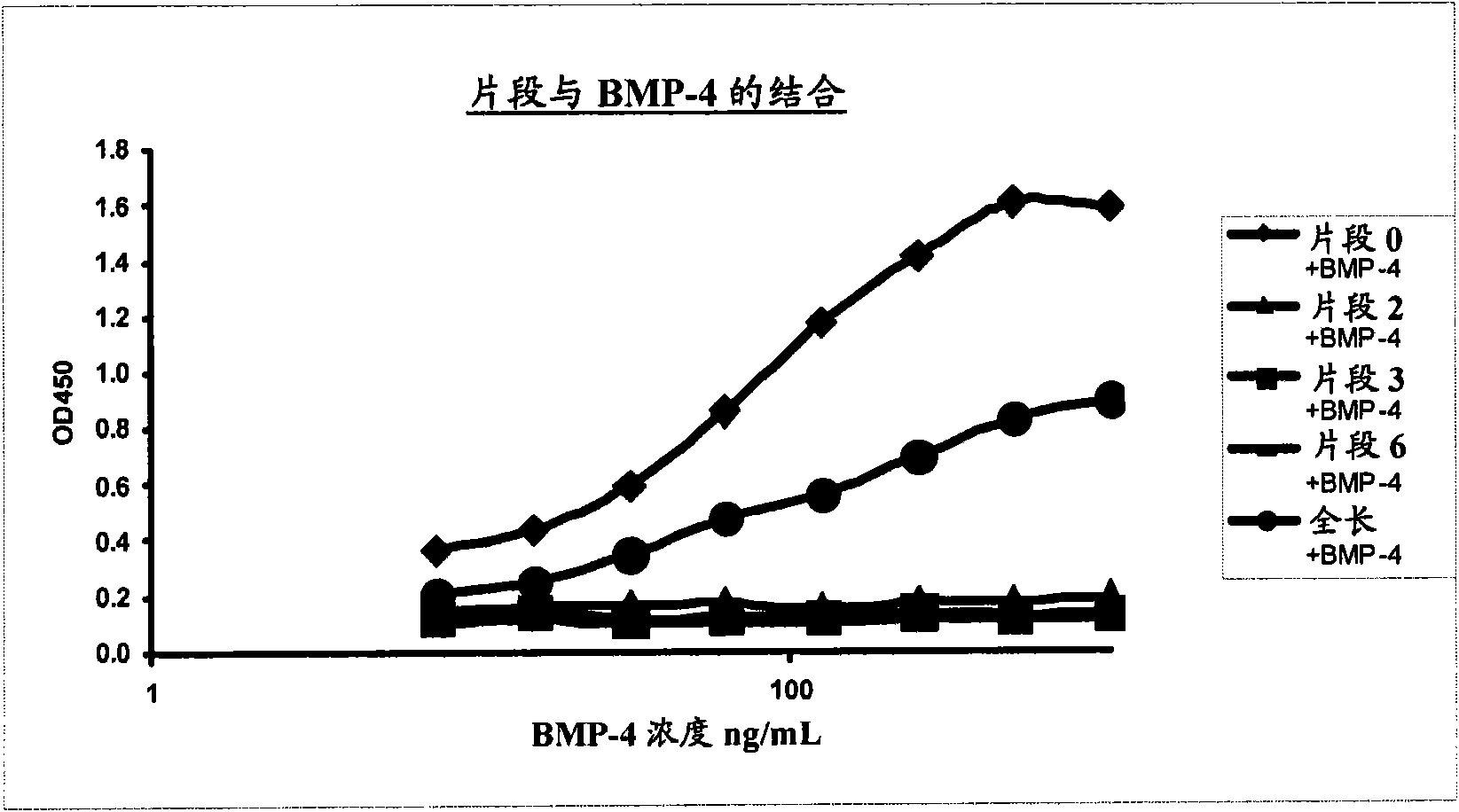
[0699] Operation example 2: In vitro interaction assays employing BMP-4 and BMP-2;
[0700] Comparing RGMA Fragment 47-168 and RGMA
[0701] Fusion protein to be tested:
[0702] RGMA-Fc
[0703] 47-168-Fc ("Fragment 0")
[0704] Detection was performed according to detection method 4 variant B above with immobilization of the fusion protein (1 mg / mL). BMP-4 and -2 binding were detected using anti-BMP-4 and anti-BMP-2 biotin antibodies, respectively.
[0705] The results are plotted in Figure 4 . Significant binding of BMP-4 and -2 to RGMA and surprisingly more pronounced binding to the 47-168 fragment was observed. BMP-2 and 4 bind with approximately equal strength in each case.
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com