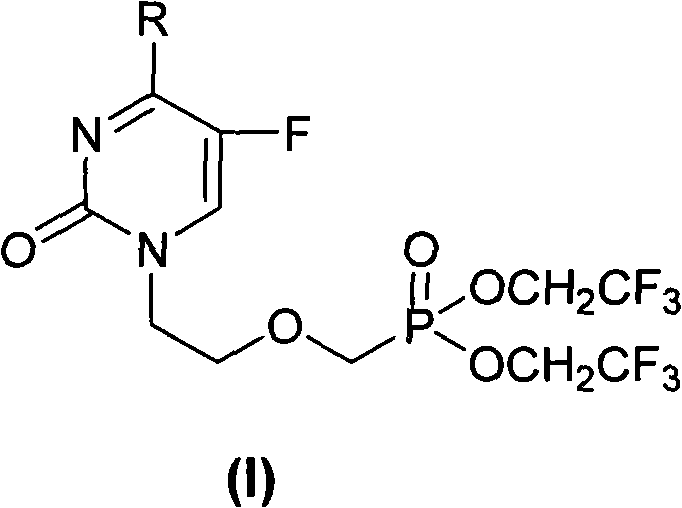
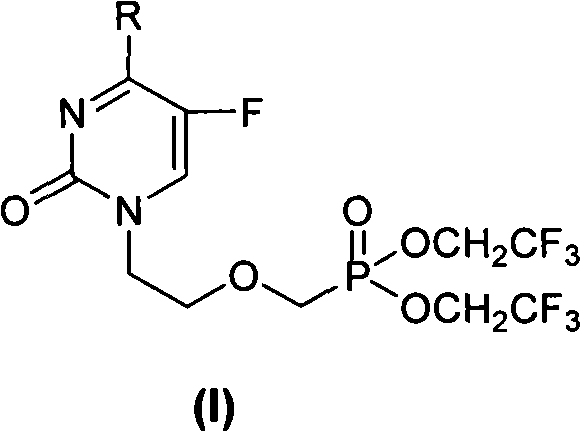
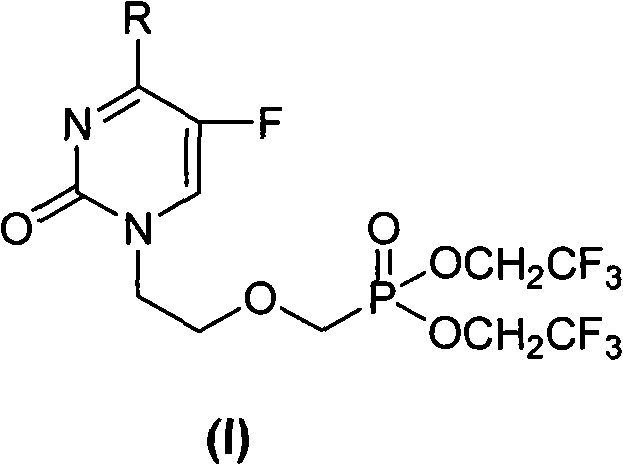
Acyclic nucleoside phosphonate compounds or pharmaceutically acceptable salts thereof, preparation method, application, intermediate compounds thereof, and medicinal composition containing same
A compound, nucleoside phosphine technology, applied in the direction of drug combination, chemical instruments and methods, compounds of group 5/15 elements of the periodic table, etc., can solve problems such as inhibition and hemorrhagic enteritis, large toxic and side effects, and patient-patient differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Preparation of 1-[bis(2,2,2-trifluoroethyl)-phosphonooxy]-5-fluoro-N 4 -Methylcytosine (compound 1)
[0085] A, 1-[bis(2,2,2-trifluoroethyl)-phosphonooxy]-5-fluoro-uracil (compound H)
[0086] The key intermediate [bis(2,2,2-trifluoroethyl)-phosphonomethyl Oxy]ethyl iodide (compound F). Dissolve 1.46g (11.2mmol) of 5-fluorouracil in 30mL of DMF, add 1.77ml (12.11mmol) of DBU and react at 80°C for 1h. Then add 4g (9.3mmol) [bis(2,2,2-trifluoroethyl)-phosphonomethoxy]ethyl iodide, and stir at 100°C for 5h. The organic solvent was evaporated, the residue was dissolved in 100 mL of dichloromethane, and the organic phase was washed with 50 mL of saturated Na 2 CO 3 aqueous solution and 50mL saturated brine. The organic phase was dried (Na 2 SO 4 ), filtered, evaporated to dryness and purified by silica gel column, developing solvent: chloroform:methanol=10:0.4 to obtain 2g of white solid, yield: 50%, mp118~120°C. 1 H-NMR (CDCl 3 )δ (ppm): 3.84 ~ 3.86 (2H,...
Embodiment 2
[0091] Example 2 Preparation of 1-[bis(2,2,2-trifluoroethyl)-phosphonooxy]-5-fluoro-N 4 -Ethylcytosine (compound 2)
[0092] Take 4.8g (10mmol) 1-[bis(2,2,2-trifluoroethyl)-phosphonooxy]-5-fluoro-4-(1,2,4-triazol-1-yl)- Pyrimidin-2-(1H)-one was added to a mixture of 5ml ethylamine and 30ml dioxane, stirred at room temperature for 1h, and evaporated to dryness. The residue was dissolved in 50ml of dichloromethane, and the organic phase was washed with 50ml of saturated sodium bicarbonate and saturated brine, and dried (MgSO 4 ), filtered and evaporated to dryness, and the residue was purified by silica gel column to obtain 2.3 g of white solid, yield: 50%, melting point 102-104°C. 1 H-NMR (CDCl 3 )δ (ppm): 1.10 (3H, t, N-CH 2 CH 3 ), 3.00~3.10 (2H, m, N- CH 2 CH 3 ), 3.66~3.69 (2H, m, N- CH 2 CH 2 -O), 3.77~3.80(2H, m, N- CH 2 CH 2 -O), 4.01 (2H, s, O- CH 2 P-), 4.20~4.23 (4H, m, 2XCF 3 CH 2 -O), 7.34 (1H, d, 6-H), 8.90 (1H, br, NH).
Embodiment 3
[0093] Example 3 Preparation of 1-[bis(2,2,2-trifluoroethyl)-phosphonooxy]-5-fluoro-N 4 -Propylcytosine (Compound 3)
[0094] According to the method described in Example 2, propylamine was used instead of ethylamine to obtain white solid compound 3, yield: 45%. 1 H-NMR (CDCl 3 )δ (ppm): 0.90 (3H, t, N-CH 2 CH 2 CH 3 ), 1.40~1.45 (2H, m, N-CH 2 CH 2 CH 3 ), 3.00~3.08 (2H, m, N- CH 2 CH 2 CH 3 ), 3.44~3.47 (2H, m, N- CH 2 CH 2 -O), 3.67~3.70 (2H, m, N-CH 2 CH 2 -O), 3.89 (2H, s, O- CH 2 P-), 4.20~4.22 (4H, m, 2XCF 3 CH 2 -O), 7.25 (1H, d, 6-H), 8.90 (1H, br, NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com