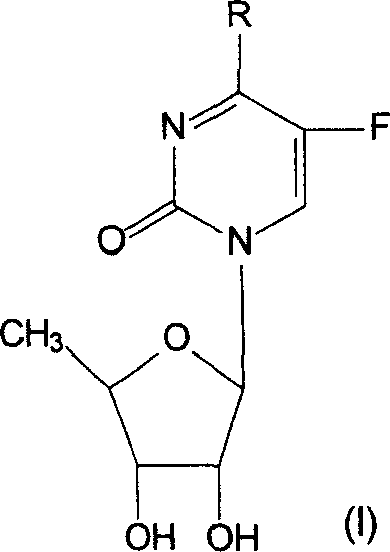
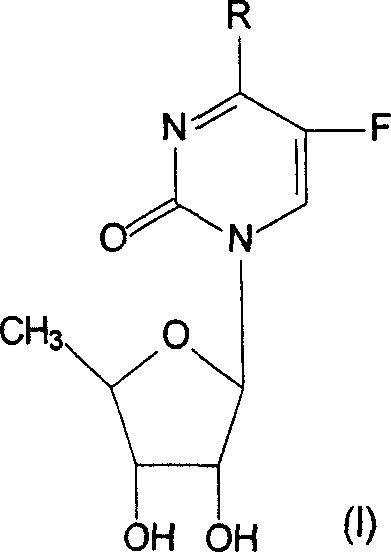
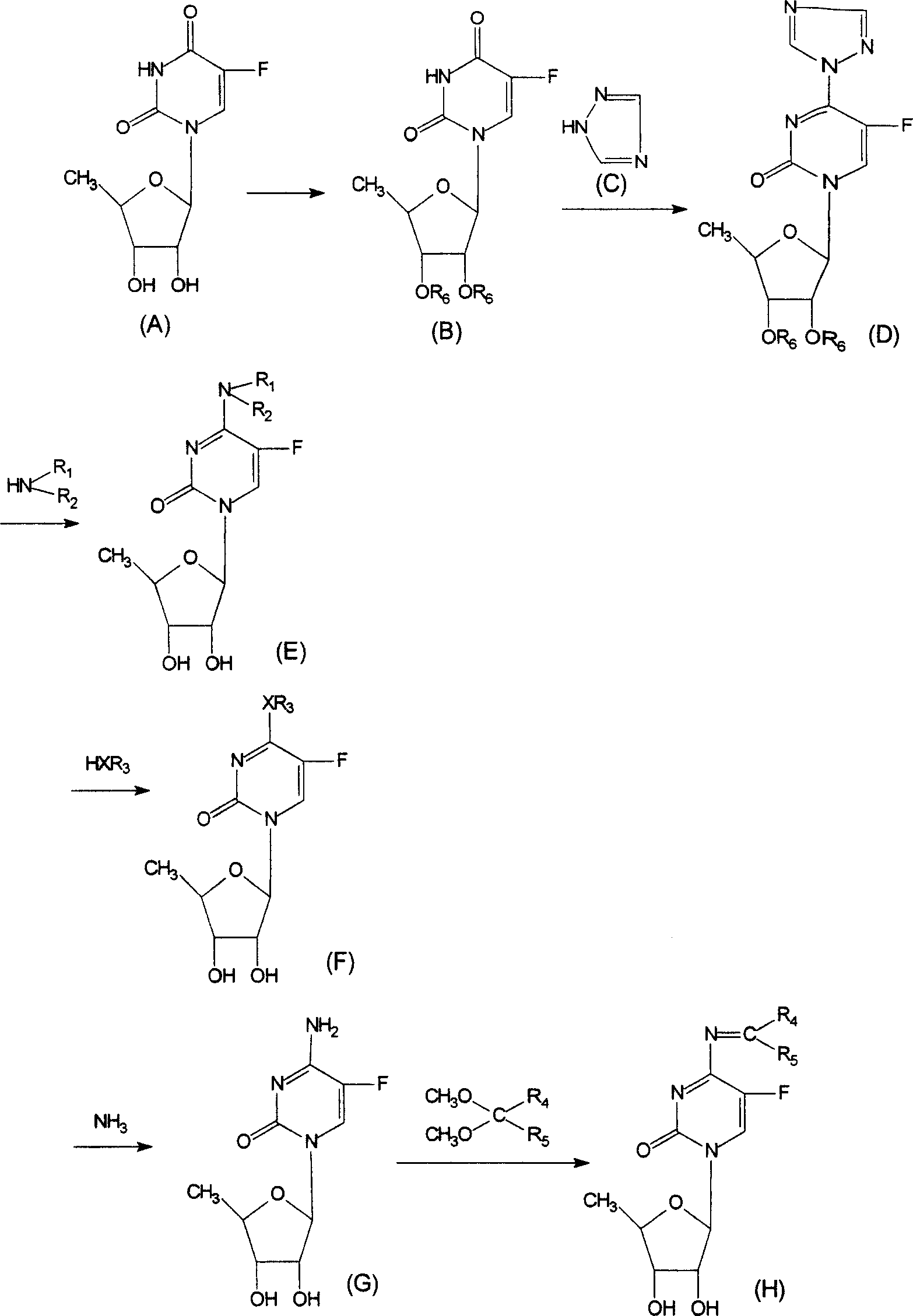
Derivative of 5-deoxy-5-fluoro cytidine and its preparation process and use
A technology of cytidine and compounds, applied in the field of 5'-deoxy-5-fluorocytidine derivatives, can solve the problems of limited application prospects and achieve good therapeutic selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of 5'-deoxy-5-fluoro-N 4 -Methylcytidine (Compound 1)
[0040] A, 2', 3'-di-O-acetyl-5'-deoxy-5-fluorouridine (compound of general formula B, wherein R 6 for acetyl)
[0041] The starting material 5'-deoxy-5-fluorouridine (compound A) was obtained by referring to the literature method (Dong Hui et al., Chinese Journal of Pharmaceutical Industry, 33:108 (2002)). 2 g (8.1 mmol) of compound A was dissolved in 40 ml of anhydrous pyridine, 1.8 ml (18 mmol) of acetic anhydride was added dropwise, and stirred overnight at room temperature. Then 5 ml of methanol was added and stirring was continued for 15 minutes, and the solution was evaporated to dryness. The solid was dissolved in 40ml of dichloromethane, washed with 40ml of 10% aqueous sodium bicarbonate solution, and the aqueous phase was extracted with 10ml×2 of dichloromethane. The dichloromethane liquids were combined, dried over anhydrous sodium sulfate, filtered, and evaporated to dryness. The residue...
Embodiment 2
[0047] Preparation of 5'-deoxy-5-fluoro-N 4 -Ethylcytidine (Compound 2)
[0048] Take 3.5g (10 mmol) 1-(2',3'-di-O-acetyl-β-D-5'-deoxyribofuranosyl)-4-(1,2,4-triazole-1- Base)-pyrimidin-2-(1H)-one was added to the mixture of 5ml ethylamine and 30ml dioxane, stirred at room temperature for 1h, and evaporated to dryness. Then 60 ml of tetrahydrofuran, 50 ml of methanol and 12.5 ml of water were added successively. Cool to 0°C, add 12.5ml of 2N sodium hydroxide solution, stir for 10 minutes and neutralize with 732-type cation exchange resin to about pH=7. The resin was filtered off, the solution was evaporated to dryness, and the residue was purified by a silica gel column to obtain 2.3 g of a white solid with a yield of 85% and a melting point of 100 to 102°C. 1 H-NMR (DMSO-d 6 )δ (ppm): 1.10 (3H, t, N-CH 2 CH 3 ), 1.28 (3H, d, 4'-CH 3 ), 3.00 to 3.10 (2H, m, N-CH 2 ), 3.66 (1H, d, 3'-H), 3.80 to 3.83 (1H, m, 4'-H), 3.99 (1H, d, 2'-H), 4.96 (1H, d, OH), 5.24 (1H, d, OH)...
Embodiment 3
[0050] Preparation of 5'-deoxy-5-fluoro-N 4 - Propylcytidine (Compound 3)
[0051] According to the method described in Implementation 2, propylamine was used instead of ethylamine to obtain white solid 5'-deoxy-5-fluoro-N 4 - Propylcytidine. 1 H-NMR (DMSO-d 6 )δ (ppm): 0.90 (3H, t, N-CH 2 CH 2 CH 3 ), 1.27 (3H, d, 4'-CH 3 ), 1.40 to 1.45 (2H, m, N-CH 2 CH 2 CH 3 ), 3.00 to 3.08 (2H, m, N-CH 2 ), 3.65 (1H, d, 3'-H), 3.80 to 3.84 (1H, m, 4'-H), 3.95 (1H, d, 2'-H), 4.96 (1H, d, OH), 5.24 (1H, d, OH), 5.71 (1H, d, 1'-H), 7.72 (1H, d, CHCF), 8.10 (1H, s, NH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com