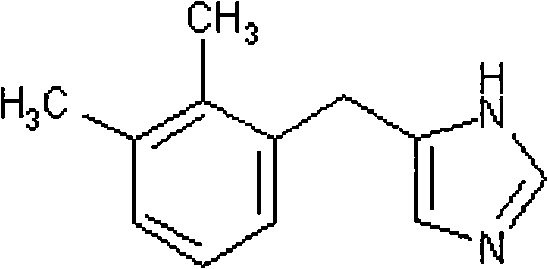
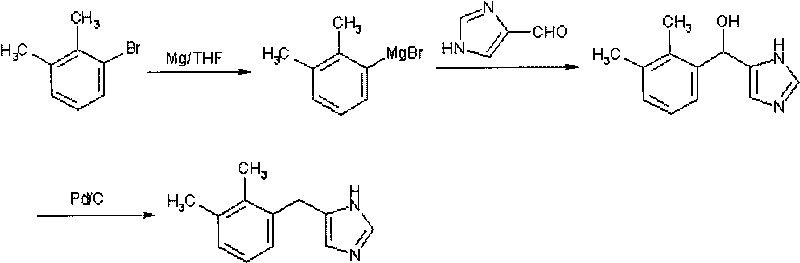
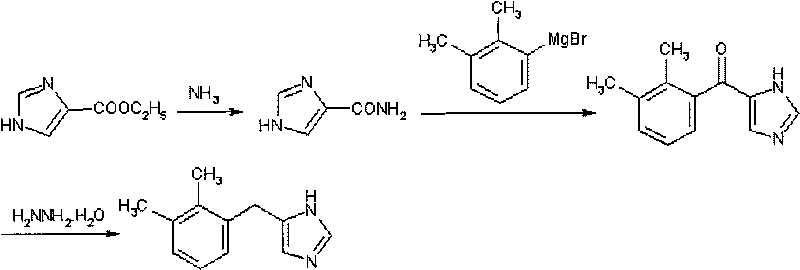
Method for preparing detomidine and the intermediate thereof
A technology for imidazole and imidazole carboxamide is applied in the field of preparing detomidine and intermediates thereof, and can solve the problems of difficulty in purchasing, high cost of detomidine raw materials, low yield of imidazole aldehyde, etc., and achieves low cost, cheap source, Easy-to-source effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 4-imidazolecarboxamide:
[0030] Add ethyl 4-imidazolate (8.5g) into water (68ml), blow in ammonia gas, heat and reflux for 6 hours, concentrate to obtain a white solid, and beat with acetone (10ml) to obtain 6.7g of a white solid, which is 4- For imidazole carboxamide, the mother liquor is recovered and merged to continue ammonolysis.
Embodiment 2
[0032] Preparation of 4-(2,3-dimethylbenzoyl)-imidazole
[0033] Magnesium turnings (45.0 g, 1.88 mol) were overlaid with 150 ml tetrahydrofuran. 2,3-Dimethylbromobenzene (220.0 ml, 1.62 mol) was dissolved in 600 ml tetrahydrofuran. Add this solution dropwise to the magnesium shavings, continue to drop slowly after the reaction is initiated, and keep refluxing. After the addition, heat to reflux until the magnesium reaction is complete, and then react for 30 minutes to obtain the Grignard reagent.
[0034] Suspend 4-imidazole carboxamide (50.0 g, 0.45 mol) in 1500 ml of pyridine, heat to 70° C., add the above-mentioned Grignard reagent dropwise, and keep the reaction for 2 hours after the dropwise addition. Cool in an ice bath to 0°C, add dropwise 1500ml of saturated ammonium chloride solution to terminate the reaction, and control the internal temperature at 5°C. The obtained solid was filtered, the filtrate was separated into layers, and the aqueous layer was extracted th...
Embodiment 3
[0036] Preparation of 4-(2,3-dimethylbenzoyl)-imidazole:
[0037] Magnesium turnings (72.0 g, 3.00 mol) were overlaid with 240 ml tetrahydrofuran. 2,3-Dimethylbromobenzene (350.0 ml, 2.58 mol) was dissolved in 800 ml tetrahydrofuran. Add this solution dropwise to the magnesium shavings, continue to drop slowly after the reaction is initiated, and keep refluxing. After the addition, heat to reflux until the magnesium reaction is complete, and then react for 30 minutes to obtain the Grignard reagent.
[0038] Suspend 4-imidazole carboxamide (50.0 g, 0.45 mol) in 1500 ml of pyridine, heat to 60° C., add the above-mentioned Grignard reagent dropwise, and keep the reaction for 2 hours after the dropwise addition. Under cooling in an ice bath, 1500 ml of saturated ammonium chloride solution was added dropwise to terminate the reaction, and the internal temperature was controlled at 5°C. The obtained solid was filtered, the filtrate was separated into layers, and the aqueous layer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com