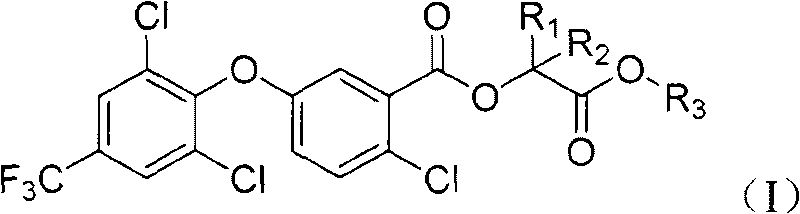
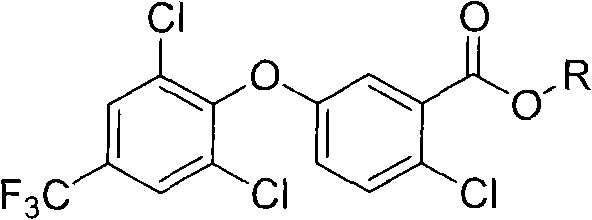
2-chloro-benzoate compound and application thereof
A technology of ester compounds and chlorobenzoic acid, which can be used in applications, organic chemistry, biocides, etc., and can solve problems such as uninvolved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: the synthesis of compound 2
[0068]
[0069] Add 25 grams of S-lactic acid (0.2 mole), methanol (32 grams, 1 mole), and 100 milliliters of benzene in sequence in a 500 milliliter reaction flask, then add 1 milliliter of concentrated sulfuric acid, heat to reflux, and use a water separator to separate the generated water, heated to reflux for 12 hours and the reaction was basically complete. The solvent was removed under reduced pressure to obtain a black oil. Distilled under reduced pressure to obtain 13.5 g of S-lactate methyl ester as a colorless liquid with a yield of 65%.
[0070]
[0071] In a 250 ml reaction flask, 3.47 grams of 2-chloro-5-(2,6-dichloro-4-(trifluoromethyl)phenoxy)benzoic acid (9 mmoles, prepared according to US4388472 method), Dichloromethane (30 ml), 2.62 g (18 mmol) of oxalyl chloride was added dropwise, and then 1 drop of DMF was added, stirred at room temperature for 5 hours, and desolvated under reduced pressure to obta...
Embodiment 2
[0074] Embodiment 2: the synthesis of compound 3
[0075]
[0076] 1.61 g (15.5 mmol) of S-methyl lactate, 2.98 g (15.53 mmol) of p-toluenesulfonyl chloride and dichloromethane (50 ml) were added successively in a 100 ml reaction flask, and 1.88 g of triethylamine ( 18.6 mmol), stirred at room temperature for 24 hours, and precipitated under reduced pressure. , the residue was purified by column chromatography (eluent was ethyl acetate:petroleum ether=1:5), to obtain 1.99 g of S-(2-p-toluenesulfonate)-methyl propionate, colorless oil, yield 50%.
[0077] Add 0.77 g (2 mmol) of 2-chloro-5-(2,6-dichloro-4-(trifluoromethyl)phenoxy)benzoic acid, N,N-di Methylformamide (20 ml), 0.42 g (3 mmol) of potassium carbonate, and 0.57 g (2 mmol) of S-(2-p-toluenesulfonyl)-propionic acid methyl ester were stirred at room temperature for 1 hour. The reaction solution was poured into 50 ml of water, extracted with 200 ml of ethyl acetate, the organic layer was washed with water and satur...
Embodiment 3
[0078] Embodiment 3: the synthesis of compound 4
[0079]
[0080] Add 0.81 g (2 mmol) of 2-chloro-5-(2,6-dichloro-4-(trifluoromethyl)phenoxy)benzoyl chloride, S-lactate ethyl Ester 0.26 g (2.2 mmol, obtained with reference to the preparation method of S-lactate methyl ester in Example 2) and methylene chloride (20 ml), was added dropwise with 0.24 g of triethylamine (2.4 mmol), stirred at room temperature for 1 hour . The reaction solution was poured into 20 ml of water, extracted with 150 ml of ethyl acetate, the organic layer was washed successively with saturated sodium carbonate solution, water, and saturated brine, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and the residue was purified by column chromatography (The eluent was ethyl acetate:petroleum ether=1:20), and 0.74 g of compound 4 was obtained as a yellow oil, with a yield of 76%. The normalized content of S-body (retention time: 5.790min) was 99.9%, and no R-body was detected...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com