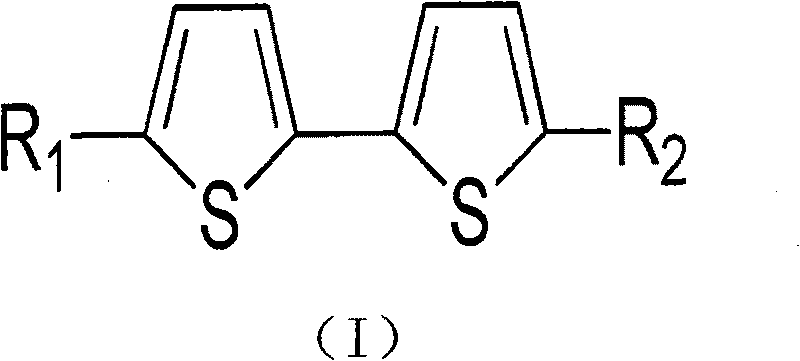
Bithiophene compound and pharmaceutical composite and application thereof
A bithiophene compound technology, applied in organic chemistry, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of complex components, difficult separation, and no bithiophene compounds with antibacterial activity, and achieve good antibacterial activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of Compound 1-10:
[0045] 1. Extraction and separation:
[0046] 20kg of the whole herb of Xinjiang blue thorn head, dried and crushed, refluxed with 95% industrial ethanol for 3 times, 4 hours each time, the extract was concentrated under reduced pressure to obtain an extract, which was dissolved in water and then extracted 3 times with petroleum ether. Extracted three times with ethyl acetate, concentrated under reduced pressure to obtain the extract, and finally extracted three times with n-butanol saturated with water; the ethyl acetate part was eluted with a gradient of chloroform-methanol solvent system to obtain six fractions of Fr1-Fr6, Fr2 The four fractions of -Fr5 were separated by repeated silica gel column chromatography, the eluent was petroleum ether: acetone (100:0-50:50) gradient elution, and the gel LH-20 was chromatographically separated, and the eluent was chloroform: Methanol (50:50) and repeated RP-18 reverse-phase silica gel column c...
Embodiment 2
[0059] Tablet: Compound 1 obtained in Example 1 10mg, lactose 180mm, starch 55mg, magnesium stearate 5mg;
[0060] Preparation method: mix compound 1, lactose and starch, wet evenly with water, sieve the wet mixture and dry it, then sieve it, add magnesium stearate, and then press the mixture into tablets, each tablet weighs 250 mg, and the compound content is 10 mg.
Embodiment 3
[0062] Ampoule: 22 mg of the compound obtained in Example 1, 10 mg of sodium chloride;
[0063] Preparation method: Dissolve compound 2 and sodium chloride in an appropriate amount of water for injection, filter the resulting solution, and fill it into an ampoule bottle under sterile conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com