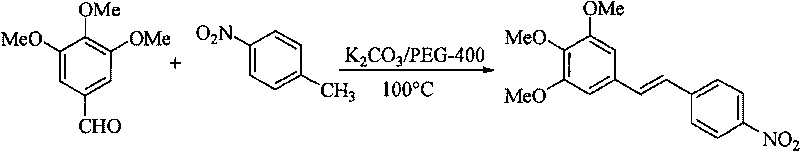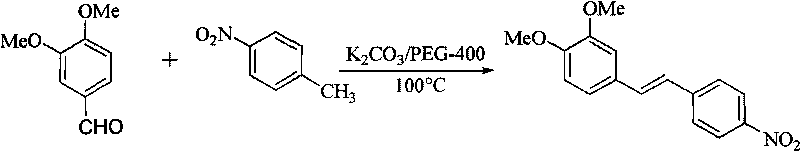Synthesis of 1,2-diarylethene compound
A technology of diarylethenes and aromatic compounds, applied in 1 field, can solve the problems of toxic organic solvents, long reaction time, high price and the like, and achieve the effects of easy purification, short reaction time and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Synthesis of (E)-3,4,5-trimethoxy-4'-nitrostilbene
[0020]
[0021] Take 1.96g (10.00mmol) 3,4,5-trimethoxybenzaldehyde, 1.37g (10.00mmol) p-nitrotoluene, 2.76g (20.00mmol) anhydrous potassium carbonate, 1.0mL PEG-400, add to In a 50mL reaction flask, react at 100°C for 3.0 hours under electromagnetic stirring, stop heating, cool to room temperature, add water and filter with suction to obtain 2.40g of the target product as a brown-yellow solid, yield: 85%. Melting point: 192~194℃;
[0022] 1 HNMR (300MHz, CDCl 3 )δ: 3.89 (s, 3H, OCH 3 ), 3.93(s, 6H, 2OCH 3 ), 6.77 (s, 2H, 2, 6-Ar-H), 7.07 (d, 1H, J=16.2Hz, -CH=CH-), 7.17 (d, 1H, J=16.2Hz, -CH=CH -), 7.64(d, 2H, 2', 6'-Ar-H), 8.24(d, 2H, 3', 5'-Ar-H);
[0023] 13 CNMR (75MHz, CDCl 3 )δ: 56.2, 60.9, 104.4, 124.1, 125.7, 126.7, 131.8, 133.3, 139.2, 143.8, 146.7, 153.6;
[0024] IRv KBr max cm -1 : 3066, 2928, 2831, 1633, 1587, 1503, 1454, 1329, 1236, 1122, 980;
[0025] Elemental analysis (%): C 17 h 17...
Embodiment 2
[0027] Synthesis of (E)-3,4-dimethoxy-4'-nitrostilbene
[0028]
[0029] Get 1.66g (10.00mmol) 3,4-dimethoxybenzaldehyde, 1.37g (10.00mmol) p-nitrotoluene, 2.76g (20.00mmol) anhydrous potassium carbonate, join in the reaction flask of 50mL, add 1.0mL PEG-400, reacted at 100°C for 3.0 hours under electromagnetic stirring, stopped heating, cooled to room temperature, added water and filtered with suction to obtain 2.60g of the target product as a brown-yellow solid, yield: 81%; melting point: 131.3~132.5°C;
[0030] 1 HNMR (300MHz, CDCl 3 )δ: 3.3.92 (s, 3H, -OCH 3 ), 3.96 (s, 3H, -OCH 3 ), 6.90 (d, 1H, J=8.5Hz, 5-Ar-H), 7.03 (d, 1H, J=16.2Hz, -CH=CH-), 7.09 (d, 1H, 6-Ar-H) , 7.09 (s, 1H, 2-Ar-H), 7.24 (d, 1H, J=16.2Hz, -CH=CH-), 7.61 (d, 2H, J=8.7Hz, 2′, 6′-Ar -H), 8.22(d, 2H, 3', 5'-Ar-H);
[0031] 13 CNMR (75MHz, CDCl 3 )δ: 56.0, 109.2, 111.4, 120.9, 124.1, 124.3, 126.5, 129.4, 133.2, 144.2, 149.4, 150.1;
[0032] IR(KBr)vmax / cm -1 : 1508, 1462, 1259, 1633, 1587,...
Embodiment 3
[0035] Synthesis of (E)-4-methoxy-4'-nitrostilbene
[0036]
[0037] Take 1.36g (10.00mmol) p-methoxybenzaldehyde, 1.37g (10.00mmol) p-nitrotoluene, 2.76g (20.0mmol) anhydrous potassium carbonate, join in 50mL reaction flask, then add 1.0mL PEG- 400, react at 70°C for 1 hour under electromagnetic stirring, then adjust to 100°C, react for 3 hours, stop heating, cool to room temperature, add water and filter with suction to obtain a yellow solid crude product, wash the solid with methanol to obtain 0.76g of the target product, the yield : 30%; Melting point: 130.0~131.8℃;
[0038] 1 HNMR (300MHz, CDCl 3 )δ: 3.85(s, 3H, OCH 3 ), 6.93 (d, 2H, 3, 5-Ar-H), 7.00 (d, 1H, J=16.6Hz, -CH=CH-), 7.20 (d, 1H, J=16.6Hz, -CH=CH -), 7.49 (d, 2H, 2, 6-Ar-H), 7.84 (d, 2H, 2', 6'-Ar-H), 8.20 (d, 2H, 3', 5'-Ar-H );
[0039] 13 CNMR (75MHz, CDCl 3 )δ: 54.3, 113.3, 123.1, 125.4, 127.3, 127.9, 131.9, 143.2, 145.4, 159.2;
[0040] IRv KBr max cm -1 : 3436, 2919, 1586, 1507, 1333 (NO 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com