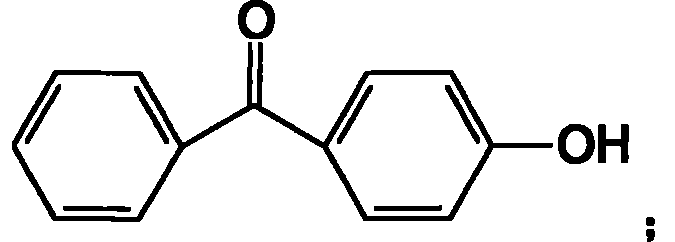
Process for preparing high-pure 4-hydroxybenzophenone
A hydroxybenzophenone, high-purity technology, applied in the direction of condensation to prepare carbonyl compounds, organic chemistry, etc., can solve the problems of low recovery rate, insufficient product yield and purity, high cost, etc., to achieve increased purity and yield , Conducive to recycling and application, and the effect of reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
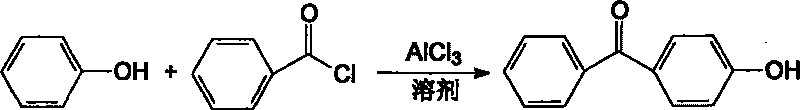
[0022] In the 1000L reactor, add chlorobenzene 200KG and aluminum trichloride 426KG (3190mol). Start stirring, and add dropwise a mixed solution of 200KG (2127mol) of phenol and 100KG of chlorobenzene at 20°C to 25°C. After the addition is complete, stir for 30 minutes, and add 314KG (2235mol) of benzoyl chloride dropwise. Slowly raise the temperature, react at 40°C-45°C for 2 hours, and react at 60°C-70°C for 1 hour.
[0023] Slowly add the above reaction liquid into 1800KG cold water, and stir at 20°C to 30°C for 2 hours. Centrifuge, wash the solid with water until neutral, and dry to obtain crude 4-hydroxybenzophenone. Then recrystallized with 2 times the amount of crude toluene to obtain 376.8 kg of white 4-hydroxybenzophenone with a yield of 89.5% and a purity of 99.5% (HPLC value). The liquid is separated into the water layer, the oil layer is washed with water until neutral, dried with anhydrous calcium chloride and then distilled to recover chlorobenzene for recycli...
Embodiment 2
[0025] In the 1000L reactor, add chlorobenzene 200KG and aluminum trichloride 426KG (3190mol). Start stirring, and add dropwise a mixed solution of 200KG (2127mol) of phenol and 100KG of chlorobenzene at 20°C to 25°C. After the addition is complete, stir for 30 minutes, and add 448KG (3190mol) of benzoyl chloride dropwise. Slowly raise the temperature, react at 40°C-45°C for 2 hours, and react at 60°C-70°C for 1 hour.
[0026] Slowly add the above reaction liquid into 1800KG cold water, and stir at 20°C to 30°C for 2 hours. Centrifuge, wash the solid with water until neutral, and dry to obtain crude 4-hydroxybenzophenone. Then recrystallize with 2 times the amount of crude toluene to obtain 347.1 KG of white 4-hydroxybenzophenone with a yield of 82.5% and a purity of 98.5% (HPLC value). The liquid is separated into the water layer, the oil layer is washed with water until neutral, dried with anhydrous calcium chloride and then distilled to recover chlorobenzene for recyclin...
Embodiment 3
[0028] In the 1000L reactor, add chlorobenzene 200KG and aluminum trichloride 312KG (2337mol). Start stirring, and add dropwise a mixed solution of 200KG (2127mol) of phenol and 100KG of chlorobenzene at 20°C to 25°C. After the addition is complete, stir for 30 minutes, and add 314KG (2235mol) of benzoyl chloride dropwise. Slowly raise the temperature, react at 40°C-45°C for 2 hours, and react at 60°C-70°C for 1 hour.
[0029] Slowly add the above reaction liquid into 1800KG cold water, and stir at 20°C to 30°C for 2 hours. Centrifuge, wash the solid with water until neutral, and dry to obtain crude 4-hydroxybenzophenone. Recrystallize with twice the amount of crude toluene to obtain 330.8KG of white 4-hydroxybenzophenone, with a yield of 78.6% and a purity of 98.2% (HPLC value). The liquid is separated into the water layer, the oil layer is washed with water until neutral, dried with anhydrous calcium chloride and then distilled to recover chlorobenzene for recycling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com