Perfluoralkylene-containing acrylate monomer and preparation method and application thereof
An olefin-based acrylate and monomer technology, which is applied to perfluoroolefin-based acrylate monomers and their preparation and application fields, can solve problems such as danger, and achieve the effects of less by-products and a simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
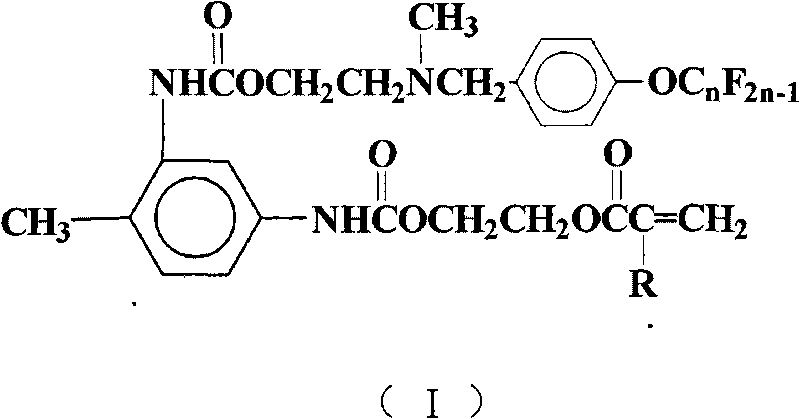
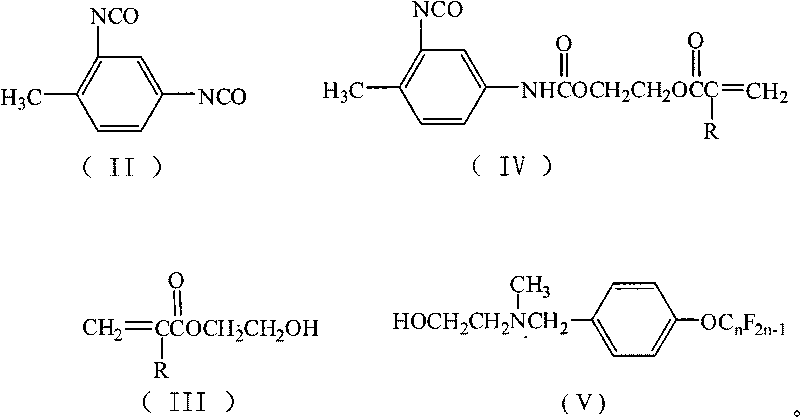
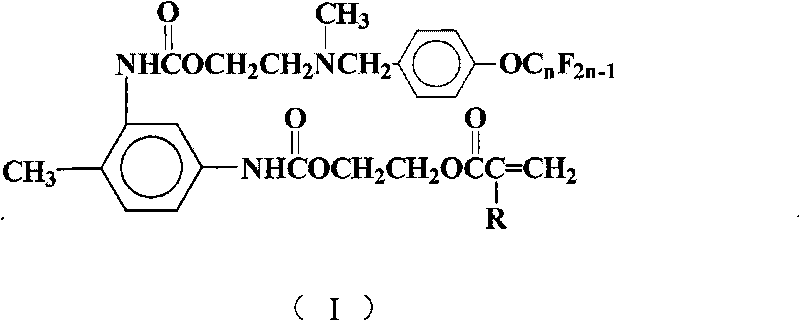
[0023] Add 15mL of 1,2-dichloroethane, 1.74g (10mmol) of toluene-2,4-diisocyanate, 0.19g (0.8mmol) of tetraethyltin, and 0.02g of hydroquinone into a 50mL four-necked flask as a polymerization inhibitor 1.30 g (10 mmol) of hydroxyethyl methacrylate was added dropwise. After the dropwise addition, TLC was used to detect the reaction progress, and the temperature was kept and stirred for 4 hours. 6.11 g (10 mmol) of N-methyl-N-hydroxyethyl-4-perfluorononenyloxybenzylamine was added dropwise. After the dropwise addition was completed, TLC was detected to track the reaction progress, and the reaction was carried out while maintaining the temperature and stirring for 5 hours. Reaction is completed, and solvent extraction is separated by silica gel column chromatography (V 二氯甲烷 :V 乙酸乙酯 :V环己烷 =5:4:1), after removing the solvent, yellow viscous paste I was obtained 1 , (n=9, R is CH 3 ), yield 63.79%, purity 99.7%.
[0024] FT-IR (cm -1 ): 3332, 2957, 2857, 2798, 1714, 1618, 1601...
Embodiment 2
[0028] Carry out the reaction according to the method of Example 1, but N-methyl-N-hydroxyethyl-4-perfluorononenyloxybenzylamine is changed to N-methyl-N-hydroxyethyl-4-perfluorohexene Base oxybenzylamine 4.61g (10mmol), other reaction conditions are with embodiment 1, obtain pale yellow paste I 2 (n=6, R is CH 3 ), yield 61.8%, purity 99.5%.
[0029] 19 F NMR δ(CDCl 3 ): -129.4(t, 3F), -127.4(s, 3F), -121.6(m, 2F), -83.3(s, 2F), -78.1(t, 1F).
Embodiment 3
[0031] Carry out reaction according to embodiment 1 method, but hydroxyethyl methacrylate is changed into hydroxyethyl acrylate 1.16g (10mmol), other reaction conditions are with embodiment 1, get yellow paste I 3 (n=9, R is H), yield 66.1%, purity 99.3%
[0032] 1 H NMR δ(CDCl 3 ): 7.83(s, 1H), 7.33(d, 2H), 7.20(s, 1H), 7.08(d, 1H), 6.85(d, 2H), 6.72(s, 1H), 6.49(s, 1H) , 6.15(s, 1H), 6.05(s, 1H), 5.59(s, 1H), 4.40(m, 4H), 4.31(t, 2H), 3.56(s, 2H), 2.74(t, 2H), 2.28(s, 3H), 2.19(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com