Preparation method of 2-formamido thienopyridine derivatives and medical uses
A technology of phenopyridine and its derivatives, which is applied in the field of preparation of antitumor pharmaceutical compositions of 2-formamidothienopyridine derivatives, and can solve the problems that there are no reports on the medical use of 2-formamidothienopyridine derivatives , to achieve good market prospects and good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 3
[0033] Preparation Example 3-Amino-N-(4-chlorobenzyl)-7-(3-methoxyphenyl)-4H-thiophene[2,3-b]pyridine-2-carboxamide (SKLB-703) preparation of
[0034] The 2-carboxamidothienopyridine derivatives used in the present invention were synthesized by the inventors themselves, with a relative molecular mass of 423.915 and a structural formula as shown in Formula II:
[0035]
[0036] The preparation method of the compound of formula II is: take p-methoxyacetophenone 1 as raw material, first react with N,N-dimethylformamide dimethyl acetal (DMF-DMA) to obtain enaminone 2, and then react with Cyanothioacetamide was ring-closed to obtain 3, and then condensed with amide 5 to obtain the target product of thienopyridines. The 3-step reaction gave the target product of formula II with a total yield of 32%.
[0037] Experimental operation and data part:
[0038] 1, the preparation of enaminone:
[0039] P-methoxyacetophenone 1 (3.19g, 21.2mmol) and N,N-dimethylformamide dimethyl aceta...
experiment Embodiment 12
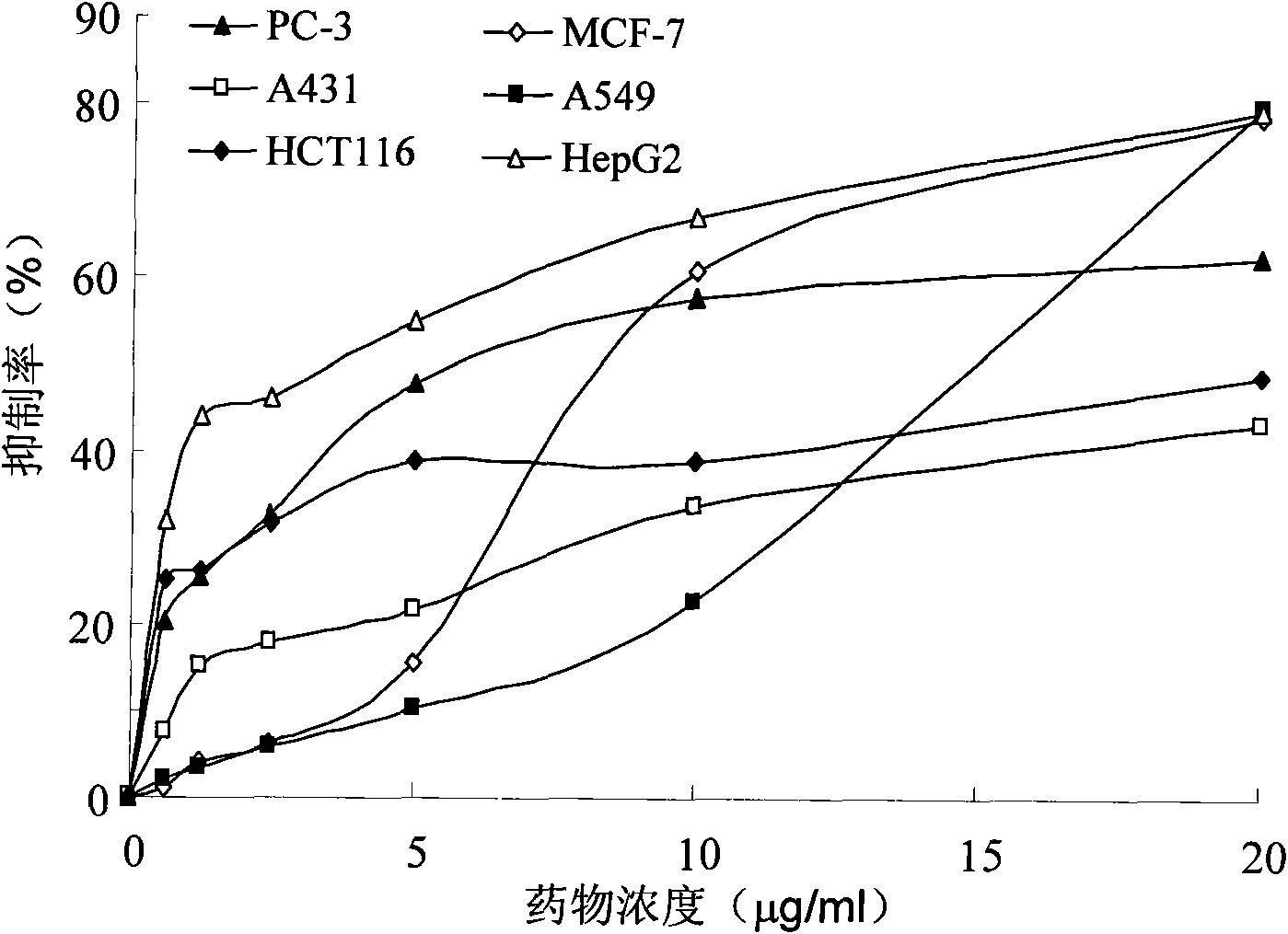
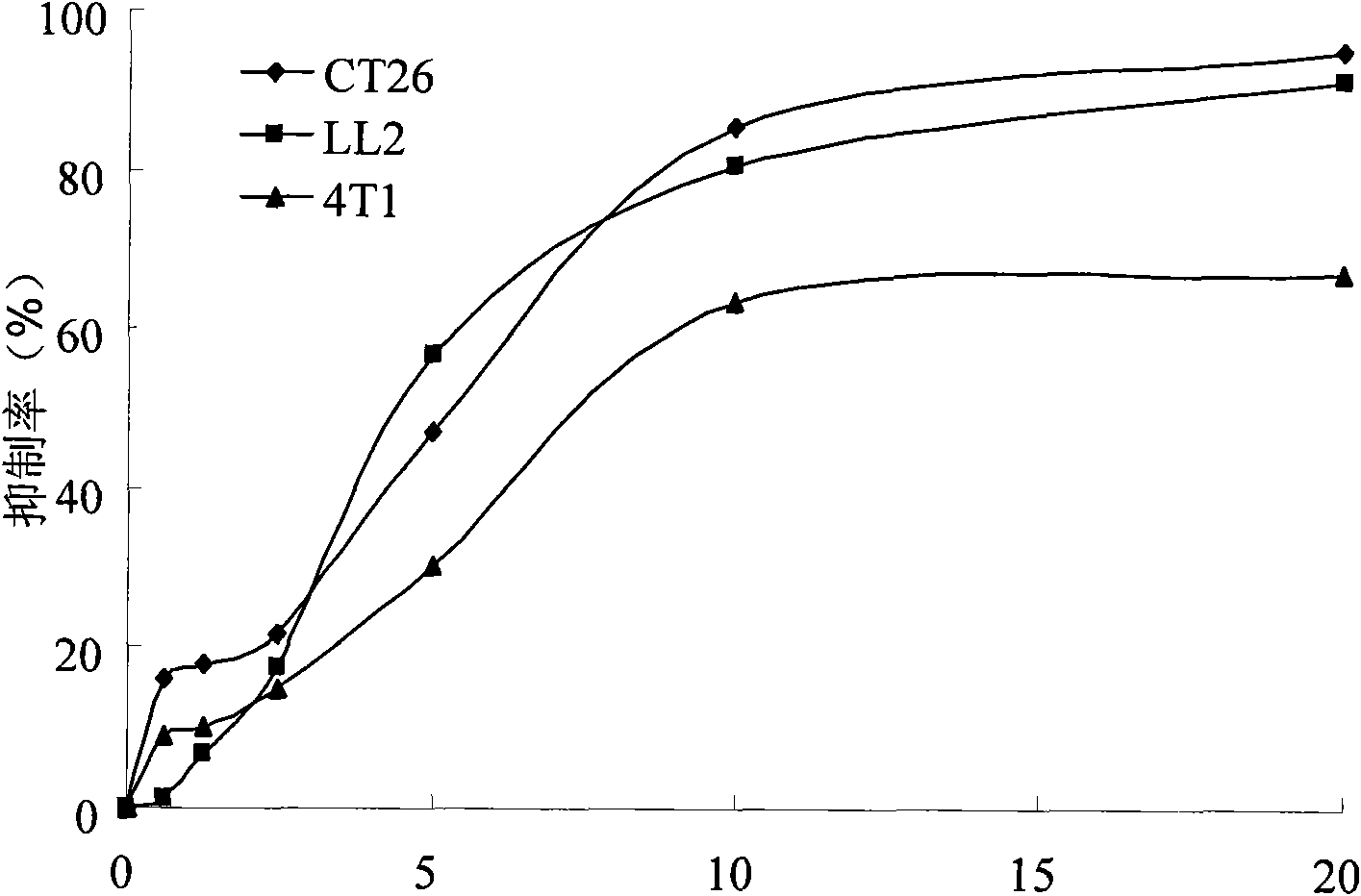
[0058] Experimental Example 12-In vitro tumor cell proliferation inhibition experiment of carboxamidothienopyridine derivatives
[0059] 1. Experimental materials
[0060] 1.1 Main reagents
[0061] RPMI-1640, DMEM, fetal calf serum, and trypsin were purchased from Gibco BRL (Invitrogen Corporation, USA), brominated thiazolyl blue tetrazolium (MTT) and dimethyl sulfoxide (DMSO) were products of Sigma Corporation (USA). 2-Carboxamidothienopyridine derivatives were purchased from Specs Company (Netherlands) and were used as chemical standards. During in vitro experiments, they were prepared as 20 mg / ml storage solution with DMSO, stored in a refrigerator at 4°C in the dark for future use, and used for complete culture before use. diluted to the desired concentration.
[0062] 1.2 Cell lines and culture
[0063] Human liver cancer cell line (HepG2), human prostate cancer cell line (PC-3), human breast cancer cell line (MCF-7), human lung cancer cell line (A549), human colon ca...
experiment Embodiment 22
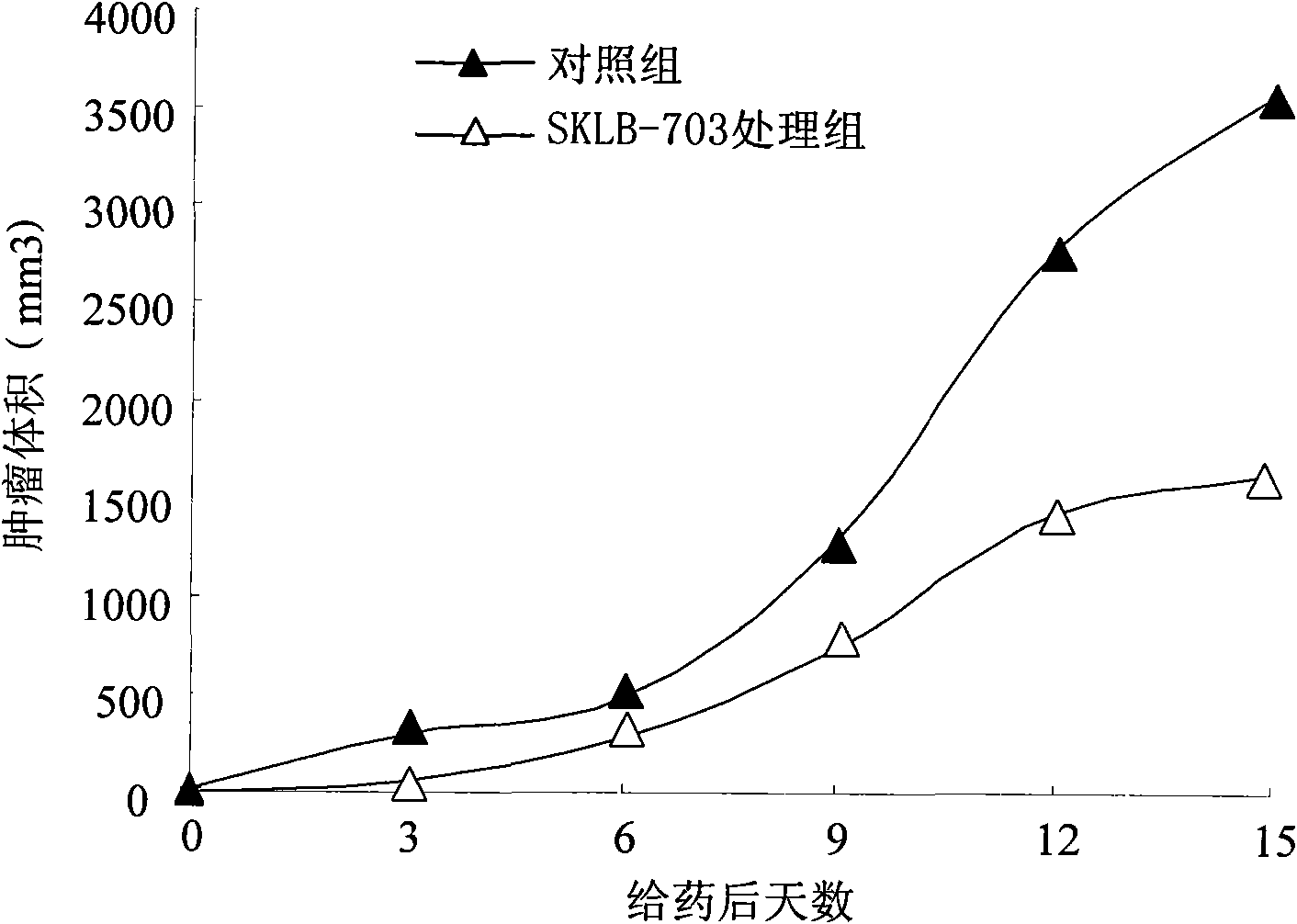
[0072] Experimental example 22-In vivo anti-tumor experiment of carboxamidothienopyridine derivatives
[0073] 1. Experimental materials
[0074] See Experimental Example 1.
[0075] 2.1 Experimental method
[0076] Establish a mouse tumor model: a mouse Lewis lung cancer model. The animal model uses female C57BL / 6 mice aged 6-8 weeks and weighing about 18-20 g. purchased from West China Animal Experiment Center, Sichuan University. Raised in the animal room of the laboratory, the animals were free to eat and drink, and the night and day alternated. The experiment began after the animals adapted to the environment. Inoculate LL / 2 cells subcutaneously into the rear flank of mice, each inoculate about 5×105 cells (0.1ml), and when the tumor grows to be palpable, divide the mice into two groups randomly: solvent control group, 60mg / kg SKLB-703 treatment group, 10 rats in each group. SKLB-703 was dissolved in 20% PEG and 10% ethanol solution and administered intraperitoneall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com