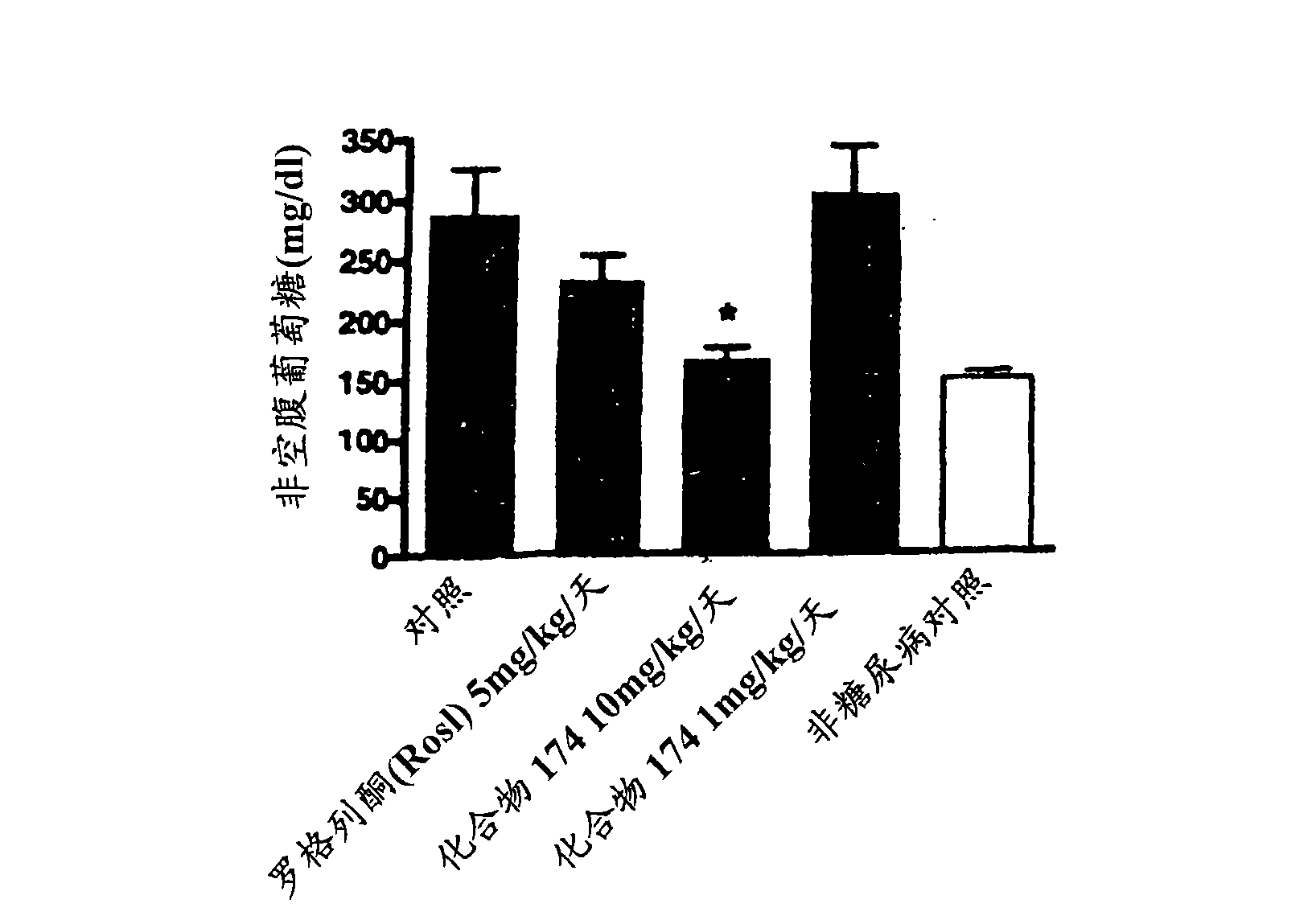
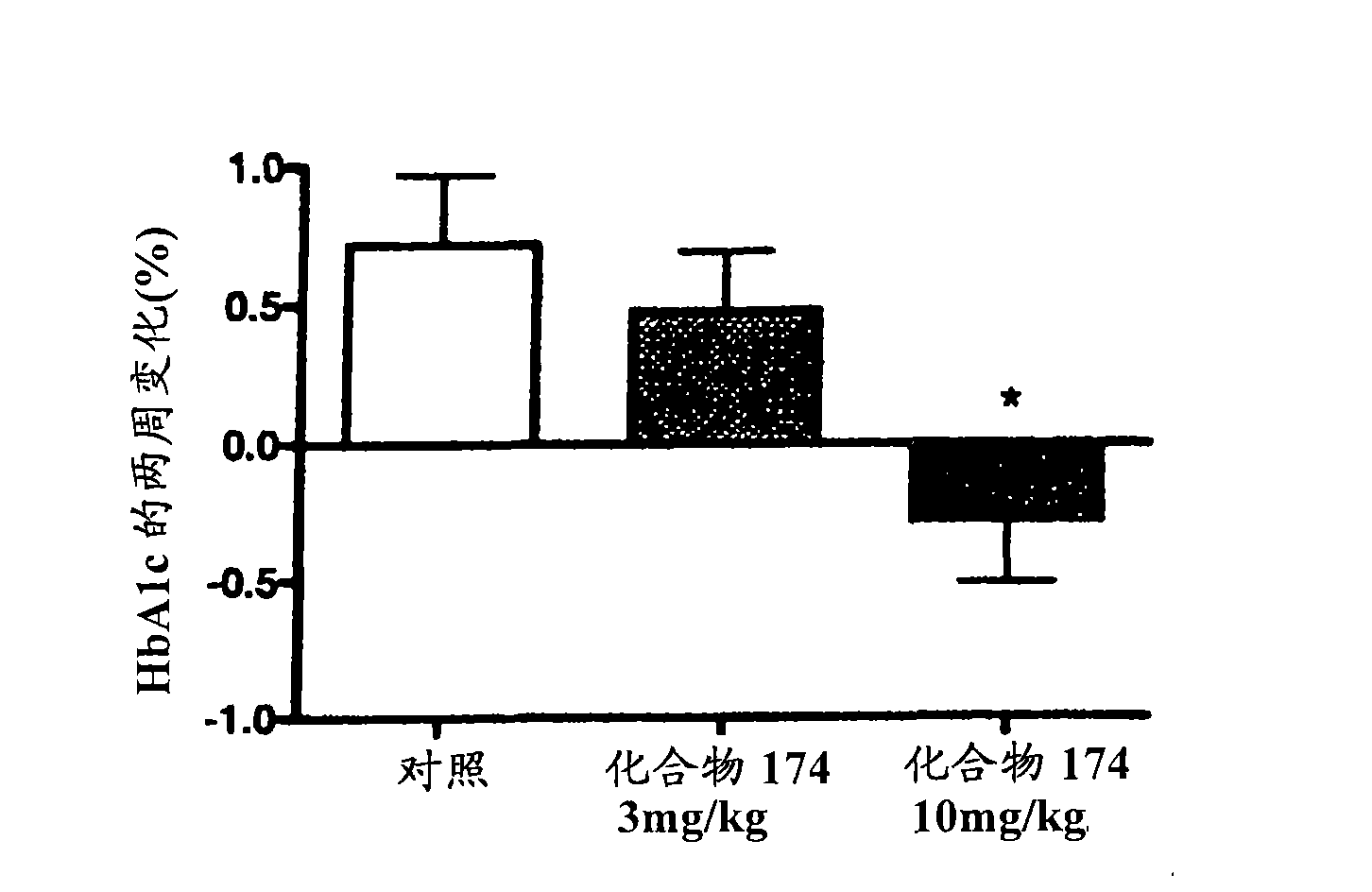
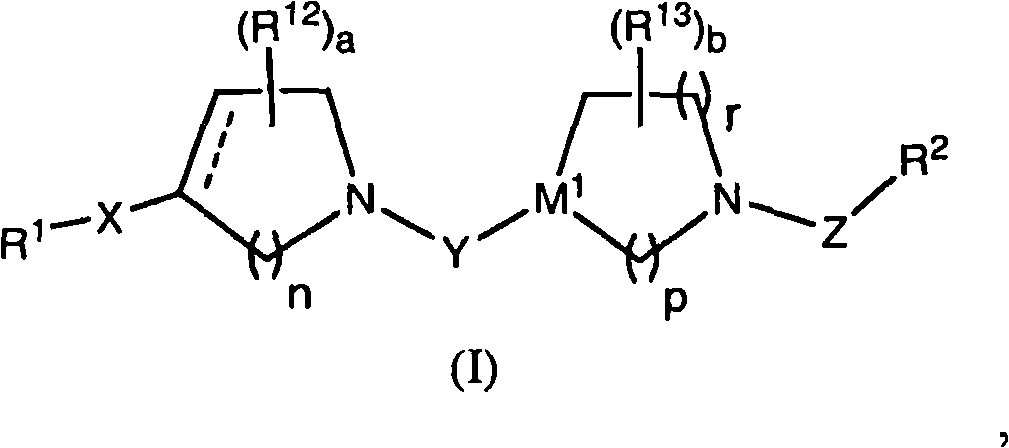
Benzimidazole derivatives and methods of use thereof
A compound and solvate technology, applied in the field of benzimidazole derivatives, can solve problems such as lactic acidosis, nausea and diarrhea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 1
[0246] Preparation of Intermediate Compound A
[0247]
[0248] Synthesis of Step 1 Compound A1
[0249]
[0250] To a solution of 2-amino-4-picoline (10.81 g, 100 mmol) in t-butanol (250 mL) was added t-BOC anhydride (26.19 g, 120 mmol). The reaction mixture was stirred at 23°C for about 15 hours, then concentrated in vacuo. The obtained crude product oil was dry-loaded on a silica gel column, and subjected to flash chromatography (eluent: 30% hexane-CH 2 Cl 2 to 0-2% acetone-CH 2 Cl 2 ), yielding 15.25 g (73.32 mmol; 73%) of compound A1 as a white solid.
[0251] Synthesis of Step 2 Compound A2
[0252]
[0253] To a solution of compound A1 (35.96 g, 173 mmol) in THF (1.4 L) was added n-BuLi (1.4M in hexane, 272 ml, 381 mmol) portionwise at -78°C over 30 min. The reaction mixture was then allowed to warm slowly and stirred at 23°C for 2 hours, an orange precipitate formed. The mixture was then cooled back to -78°C and pre-dried oxygen (through a Drierite col...
Embodiment 2
[0263] Preparation of Intermediate Compound B
[0264]
[0265] Synthesis of step 1 compound B2
[0266]
[0267] Diamine 1B (see step 1 of Example 1) (20 g, 71.1 mmol) and Et 3 CH of N (30ml, 213mmol) 2 Cl 2 (400ml) solution was cooled to 0°C in an ice-water bath. To this stirred solution was added triphosgene (14.2 g, 47.3 mmol) in portions carefully (exothermicly!) over 30 minutes. When the addition was complete, stirring was continued at 0°C for 1 hour, then at room temperature for 16 hours. The mixture was washed with 0.5N NaOH (200ml), and the organic layer was washed with anhydrous MgSO 4 Dry and concentrate in vacuo. To the semi-solid residue was added hot EtOAc (200ml) and the resulting mixture was cooled to room temperature. Filtration to obtain compound B2 (16.5g) as a white solid; the filtrate was subjected to silica gel flash chromatography [CH 2 Cl 2 -CH 3 OH(2N NH 3 )=40:1], an additional product (2.7 g) was obtained as a white solid [total yiel...
Embodiment 3
[0278] Preparation of Intermediate Compound C
[0279]
[0280] Synthesis of Step 1 Compound C1
[0281]
[0282] In access N 2 In a flask, NaH (60mg, 60% dispersion; 1.48mmol) was added to CH 3 OH (4ml). After stirring at room temperature for 30 minutes, compound B3 (400 mg, 1.23 mmol) was added, and the resulting mixture was stirred at room temperature for 16 hours. Vacuum removal of CH 3 OH, CH was added to the resulting residue 2 Cl 2 (30ml) and water (10ml). The organic layer was washed with anhydrous MgSO 4 Dry, filter and concentrate in vacuo. The resulting residue was purified using flash chromatography on silica gel, eluting with EtOAc-hexane (3:2), to afford compound C1 (0.232 g; 59%) as a white foam. ES-MS: 322.1 (MH + ; 100%).
[0283] Synthesis of Step 2 Compound C
[0284] 1N Aqueous KOH (4.82ml; 4.82mmol) was added to a solution of compound C1 in EtOH (15ml), and the resulting mixture was stirred at 80°C for 48 hours. The mixture was concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com