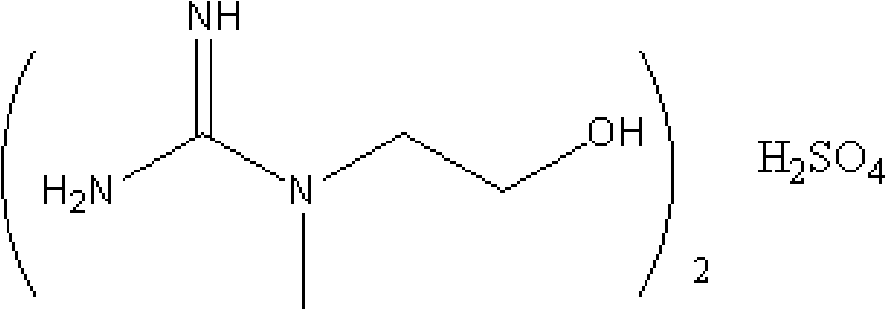
Preparation method for creatinol sulphate
The technology of inositol sulfate and concentrated sulfuric acid is applied in the field of preparation of inositol sulfate, can solve the problems of polluted environment, large consumption of organic solvent and high production cost, achieves good purity, simple and easy post-processing, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
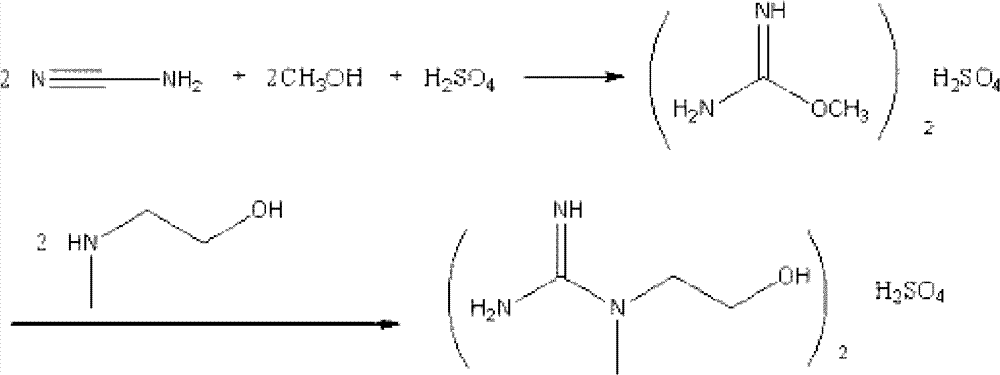
[0021] Add 77 mL (0.55 mol) of an aqueous solution containing 23 g of cyanamide and 20 mL (0.5 mol) of methanol into a 250 mL four-neck flask, add 26 g (0.26 mol) of 98% concentrated sulfuric acid dropwise, and stir at room temperature for 60 min after adding sulfuric acid. Then, 37.5 g (0.5 mol) of N-methylaminoethanol was added, and after the addition was completed, the temperature was raised at 60° C. for 5 h. After the reaction was completed, it was concentrated under reduced pressure. Add 100mL of absolute ethanol while stirring at 50°C, the formed myo-sulphate precipitates out as granular crystals, filter the precipitated crystals after cooling, wash with absolute ethanol, and dry. The yield is 60-70%. Product purity reaches 93-99%
Embodiment 2
[0023] Add 70 mL (0.5 mol) of aqueous solution containing 21 g of cyanamide and 20 mL (0.5 mol) of methanol into a 250 mL four-neck flask, add 25 g (0.25 mol) of 98% concentrated sulfuric acid dropwise, and stir at room temperature for 60 min after adding sulfuric acid. Then, 37.5 g (0.5 mol) of N-methylaminoethanol was added, and after the addition was completed, the temperature was raised at 70° C. for 4 h. After the reaction was completed, it was concentrated under reduced pressure. When the temperature is lowered to 60°C, add 200ml of acetone under stirring, and the generated myo-sulphate precipitates in the form of granular crystals. After cooling, filter the precipitated crystals, wash them with absolute ethanol, and dry them. The yield is 65-70%. The product purity reaches 93-99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com