Preparation method of transparent ceramic glitter material
A technology of scintillation materials and transparent ceramics, applied in the field of cubic phase inorganic transparent ceramic scintillation materials, can solve the problems of difficulty in avoiding the second phase, decreased luminous brightness, high hardness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] a), weigh 10g Gd 2 o 3 Soluble in HNO 3 1.8955mol / L Gd(NO 3 ) 3 Solution, weigh 15g Eu 2 o 3 Soluble in HNO 3 0.09569mol / L of Eu(NO 3 ) 3 solution;
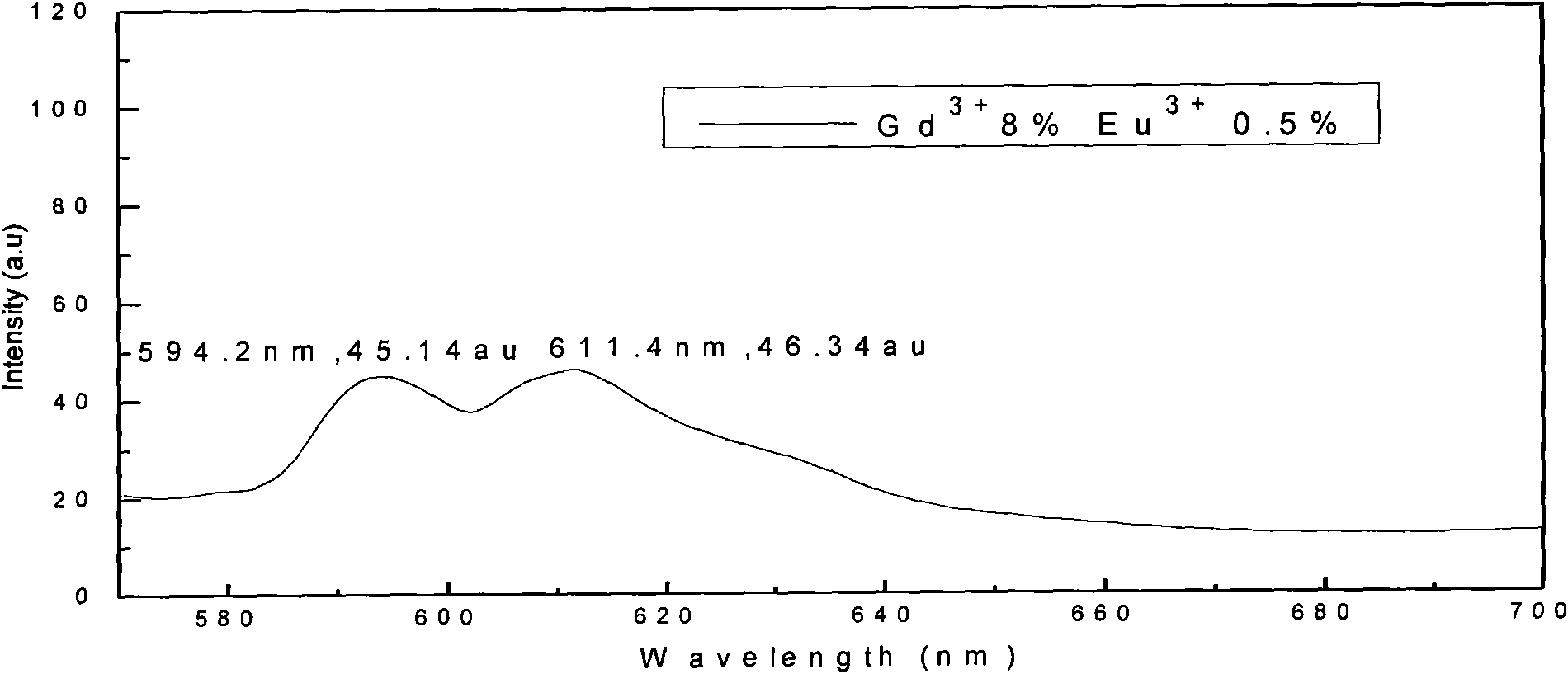
[0026] b), weigh 4.8832g of HfOCl 2 After the powder was put into a beaker and dissolved in distilled water, 0.84ml of Gd(NO 3 ) 3 solution, in which Gd 3+ The molar content of Gd 3+ and Hf 4+ 8% of the total molar content, after stirring evenly, add 1.0ml of Eu(NO 3 ) 3 solution, measure the pH value of the solution, and adjust it to 1 with nitric acid;
[0027]c) Add 0.6820g of glycine and 0.7884g of urea mixed fuel to the above solution while stirring, vigorously stir to make it fully dissolved, heat at 200°C for 0.5h to remove moisture, and burn in a muffle furnace at 450°C for 1h obtain precursors;
[0028] d) Sinter the precursor in a high temperature furnace at 800°C for 2h to obtain HfO 2 -Gd 2 o 3 :Eu 3+ Solid solution transparent ceramic scintillation material.
Embodiment 2
[0030] a), weigh 10g Gd 2 o 3 Soluble in HNO 3 1.8955mol / L Gd(NO 3 ) 3 Solution, weigh 15g Eu 2 o 3 Soluble in HNO 3 0.09569mol / L of Eu(NO 3 ) 3 solution;
[0031] b), weigh 4.6178g of HfOCl 2 After the powder was put into a beaker and dissolved in distilled water, 1.4ml of Gd(NO 3 ) 3 solution, in which Gd 3+ The molar content of Gd 3+ and Hf 4+ 13% of the total molar content, after stirring evenly, add 1.0ml of Eu(NO 3 ) 3 solution, measure the pH value of the solution, and adjust it to 1 with nitric acid;
[0032] c) Add 0.6820g of glycine and 0.7884g of urea mixed fuel to the above solution while stirring, vigorously stir to make it fully dissolved, heat at 200°C for 0.5h to remove moisture, and burn in a muffle furnace at 450°C for 1h obtain precursors;
[0033] d) Sinter the precursor in a high temperature furnace at 800°C for 2h to obtain HfO 2 -Gd 2 o 3 :Eu 3+ Solid solution transparent ceramic scintillation material.
Embodiment 3
[0035] a), weigh 10g Gd 2 o 3 Soluble in HNO 3 1.8955mol / L Gd(NO 3 ) 3 Solution, weigh 15g Eu 2 o 3 Soluble in HNO 3 0.09569mol / L of Eu(NO 3 ) 3 solution;
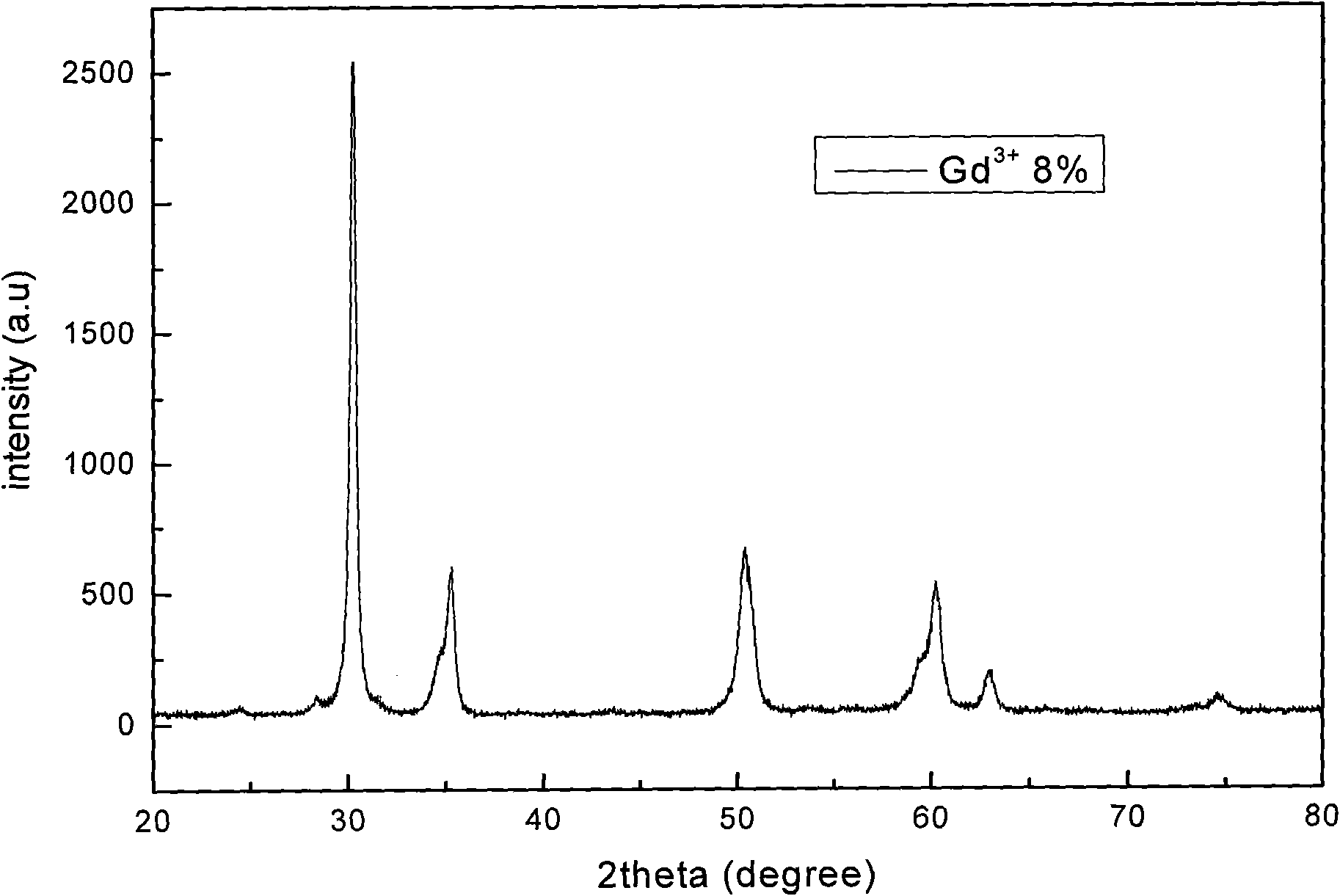
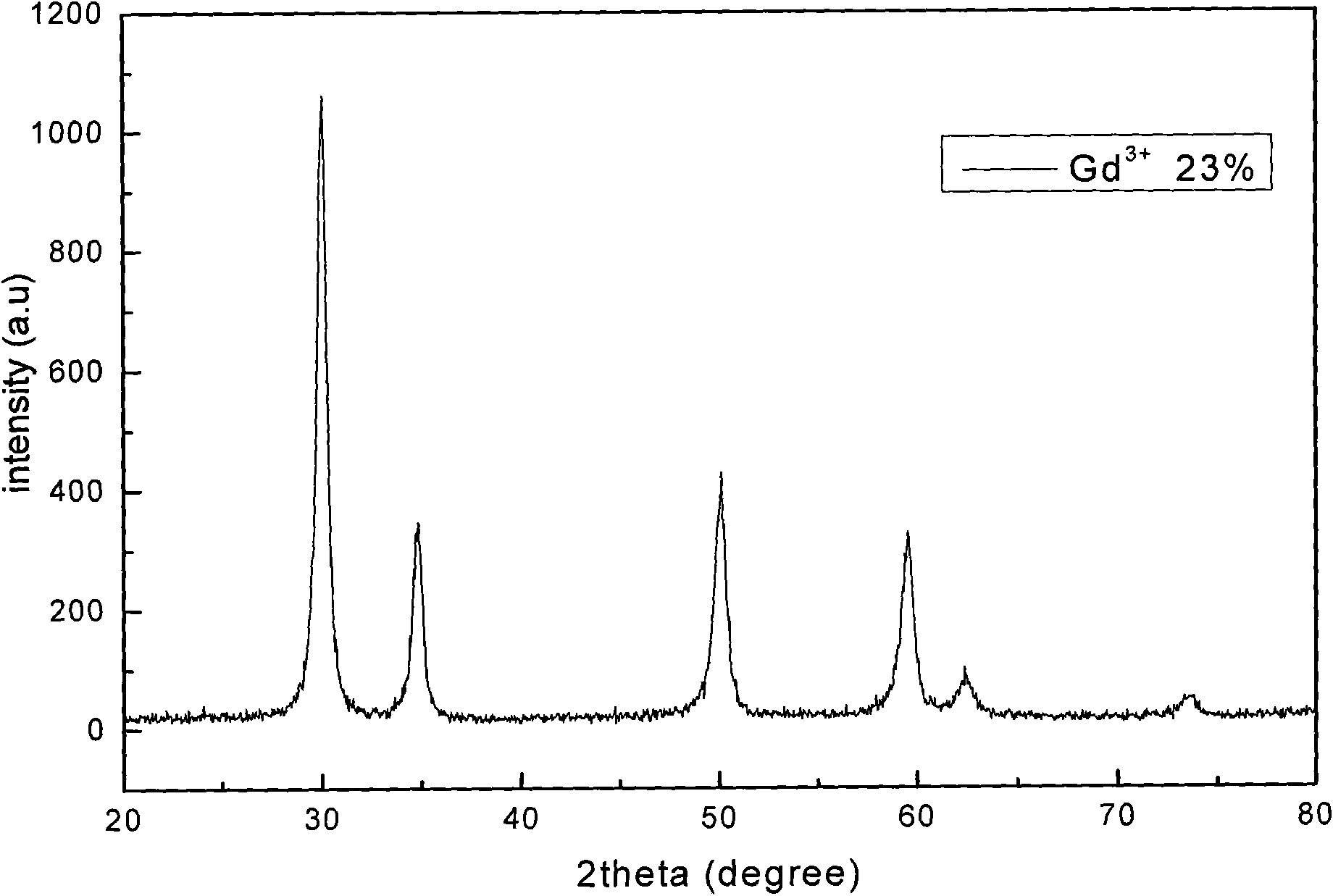
[0036] b), weigh 4.0870g of HfOCl 2 After the powder was put into a beaker and dissolved in distilled water, 2.4ml of Gd(NO 3 ) 3 solution, in which Gd 3+ The molar content of Gd 3+ and Hf 4+ 23% of the total molar content, after stirring evenly, add 1.0ml of Eu(NO 3 ) 3 solution, measure the pH value of the solution, and adjust it to 1 with nitric acid;
[0037] c) Add 0.6820g of glycine and 0.7884g of urea mixed fuel to the above solution while stirring, vigorously stir to make it fully dissolved, heat at 200°C for 0.5h to remove moisture, and burn in a muffle furnace at 450°C for 1h obtain precursors;
[0038] d) Sinter the precursor in a high temperature furnace at 800°C for 2h to obtain HfO 2 -Gd 2 o 3 :Eu 3+ Solid solution transparent ceramic scintillation material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com