Pyrazole amide derivative and application thereof
A technology of pyrazole amides and derivatives is applied in the field of agrochemical pesticide synthesis, which can solve the problems of increased consumer spending and reduced productivity, and achieve the effects of improving fat solubility and increasing insecticidal activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
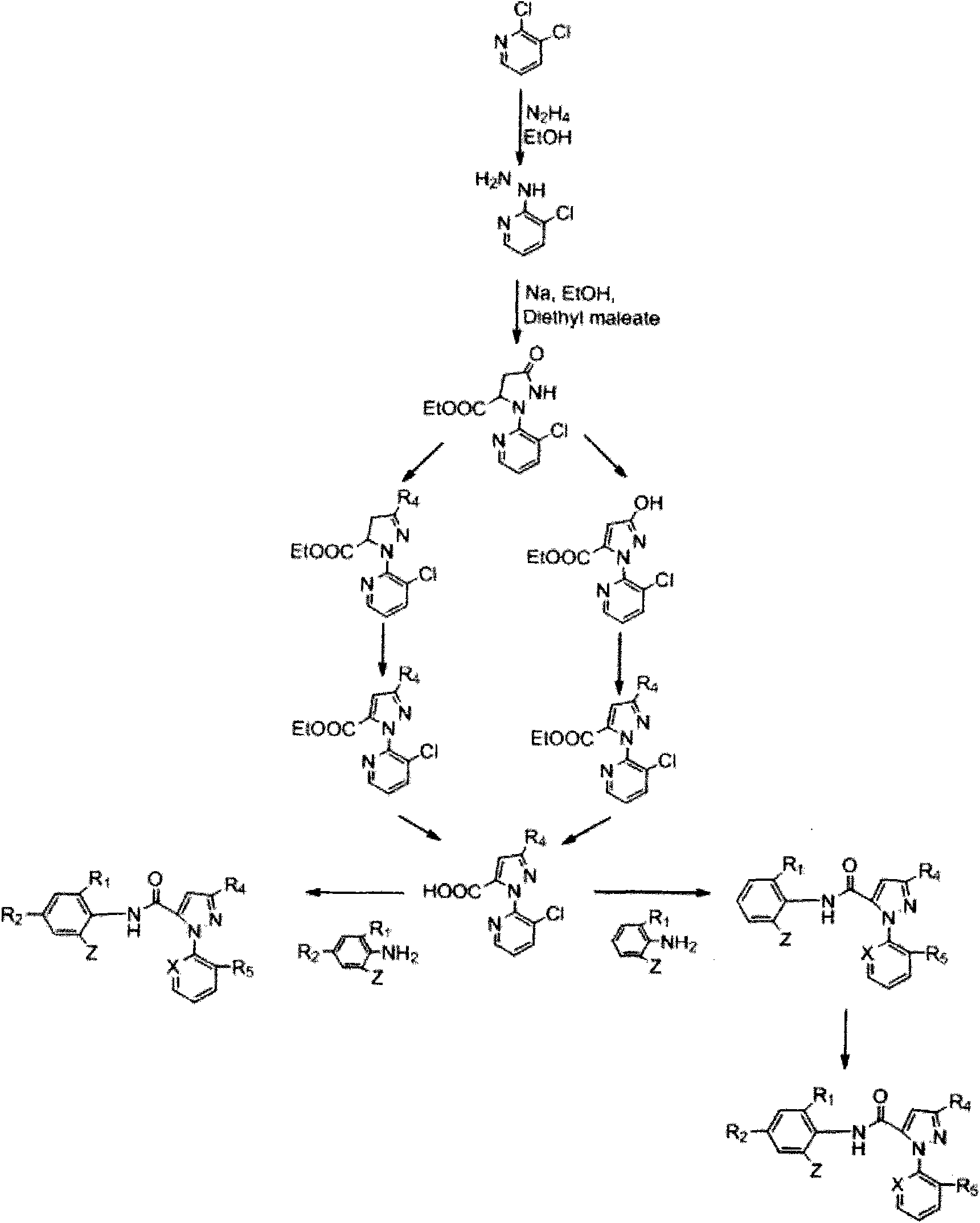
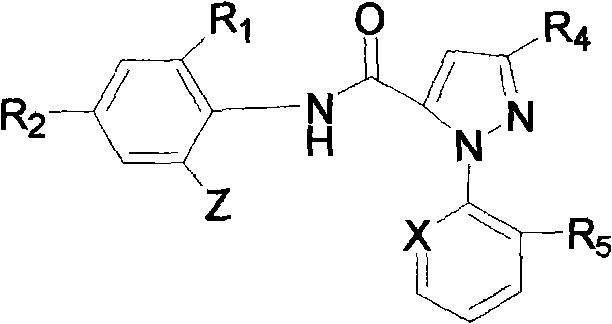
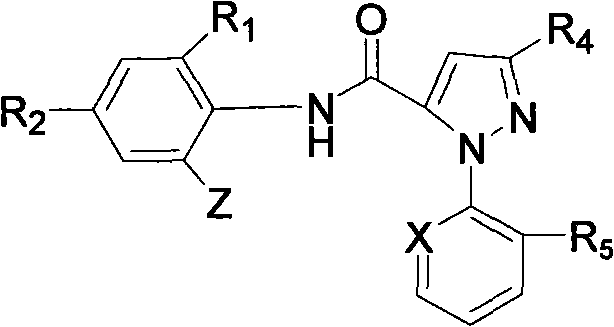
[0029] 3-Acetoxy-1-(3-chloro-2-pyridyl)-N-[4-chloro-2-methyl-6-[((methyl)amino)-carbonyl]phenyl]-1H- Synthesis of pyrazole-5-carboxamide (derivative 01):
[0030] Step A: Preparation of 3-chloro-2-hydrazinopyridine
[0031] 200 mL of 50% hydrazine hydrate was slowly added to a solution of 2,3-dichloropyridine (73.5 g, 0.5 mol) in ethanol (300 mL) at room temperature, the reaction mixture was refluxed for 36 h, and cooled to room temperature; the white needle-like solid was collected by filtration, The solid was washed with cold ethanol (3×50 mL) to obtain 67.5 g of 3-chloro-2-hydrazinopyridine.
[0032] Step B: Preparation of ethyl 1-(3-chloro-2-pyridyl)-3-pyrazolidinone-5-carboxylate
[0033] Add 200mL of ethanol to a 500mL three-neck round bottom flask, slowly add sodium metal (6.9g, 0.3mol), when all the sodium metal has reacted completely, the reaction solution is heated to reflux, and 3-chloro- 2-Hydrazinopyridine (39.82 g, 0.277 mol). After the reaction mixture was r...
Embodiment 2
[0043] 3-Acetoxy-1-(3-chloro-2-pyridyl)-N-[4-bromo-2-methyl-6-[((isopropyl)amino)-carbonyl]phenyl]-1H - Synthesis of pyrazole-5-carboxamide (derivative 15):
[0044] Step A: Preparation of 2-amino-3-methyl-5-bromobenzoic acid
[0045] Dissolve 2-amino-3-methylbenzoic acid (10g, 66mmol) in 50mL DMF, slowly add NBS (11.7g, 66mmol) to it in portions, then heat to 100°C, react for 2h, pour into 200mL ice In the water, a white solid appeared, filtered, dissolved the solid with ethyl acetate, dried, and precipitated to obtain an off-white solid, which was washed with ether to obtain 12.9 g of 2-amino-3-methyl-5-bromobenzoic acid as a white solid.
[0046] Step B: Preparation of N-isopropyl-2-amino-3-methyl-5-bromobenzamide
[0047] Dissolve 2-amino-3-methyl-5-bromobenzoic acid (3.7g, 20mmol) in 40 mL of thionyl chloride, reflux for 4 hours, remove thionyl chloride under reduced pressure, and dissolve the residue in 20 mL of tetrahydrofuran, Slowly drop into the tetrahydrofuran so...
Embodiment 3
[0051] 3-Trifluoroethoxy-1-(3-chloro-2-pyridyl)-N-[4-chloro-2-methyl-6-[((cyclopropyl)amino)-carbonyl]phenyl] - Synthesis of 1H-pyrazole-5-carboxamide (derivative 18):
[0052] Step A: Preparation of ethyl 3-trifluoroethoxy-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylate
[0053] 3-Hydroxy-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylic acid ethyl ester (1.0 g, 3.7 mmol) was dissolved in 20 mL DMF and potassium carbonate (0.76 g, 5.5 mmol) was added , heated to 100°C and added trifluoroiodoethane (0.94g, 4.4mmol) dropwise, continued reaction for 3h after dropping, diluted with water, extracted with ethyl acetate, dried, and precipitated to obtain 3-trifluoroethoxy-1- (3-Chloro-2-pyridyl)-1H-pyrazole-5-carboxylic acid ethyl ester 1.30 g.
[0054] Step B: Preparation of 3-trifluoroethoxy-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylic acid
[0055] Dissolve ethyl 3-trifluoroethoxy-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylate (1.3g, 3.7mmol) in 20mL of methanol, add hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com