Preparation method for preparing high-purity II type (+)-(s)-clopidogrel bisulfate
A clopidogrel bisulfate, high-purity technology, applied in directions such as organic chemistry, to achieve the effects of improving yield, reducing production costs, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of type I (+)-(S)-clopidogrel bisulfate
[0042]Put 130g of clopidogrel free base and 700mL of ethyl acetate into a 1L three-necked flask, stir at room temperature until dissolved and clear, add 40g of concentrated sulfuric acid dropwise, and complete the dropwise addition in 30 minutes. The reaction solution continued to stir for 2 hours, solids precipitated, filtered; washed with ethyl acetate (50mL×3), and dried in vacuo at 50°C to obtain 148g of type I (+)-(S)-clopidogrel hydrogensulfate, collected The yield is 87%, the purity is 97.4%, and the impurity C content is 1.5%.
Embodiment 2II
[0043] Preparation of Example 2 Type II (+)-(S)-clopidogrel bisulfate
[0044] Put 80g type I clopidogrel bisulfate and 120mL methanol into a 1L three-necked flask, stir at room temperature until dissolved and clarified, add 480mL of ethyl formate, stir for 6 hours, a small amount of solid precipitates, remove by filtration; put the mother liquor into a 1L three-necked flask, Cool down to 0°C, stir for 5 hours, a large amount of solid precipitates, filter, wash with ethyl formate (30mL×3), and dry in vacuum at 50°C to obtain 67g I II(+)-(S)-type clopidogrel hydrogen sulfate Salt, the refined yield is 84%, the purity is 99.8%, and the impurity C content is 0.07%.
Embodiment 3II
[0045] The preparation of embodiment 3II type (+)-(S)-clopidogrel bisulfate
[0046] Put 80g of type I clopidogrel bisulfate and 120mL of methanol into a 1L three-necked flask, stir at room temperature until dissolved and clarified, add 680mL of ethyl acetate, stir for 30 minutes, a small amount of solid precipitates, remove by filtration; put the mother liquor into a 1L three-necked flask, Cool down to -15°C and stir for 7 hours, a large amount of solid precipitated, filtered, washed with ethyl acetate (30mL×3), and dried in vacuum at 50°C to obtain 65g of type III (+)-(S)-clopidogrel hydrogen sulfate Salt, the refined yield is 81%, the purity is 101.1%, and the impurity C content is 0.09%.
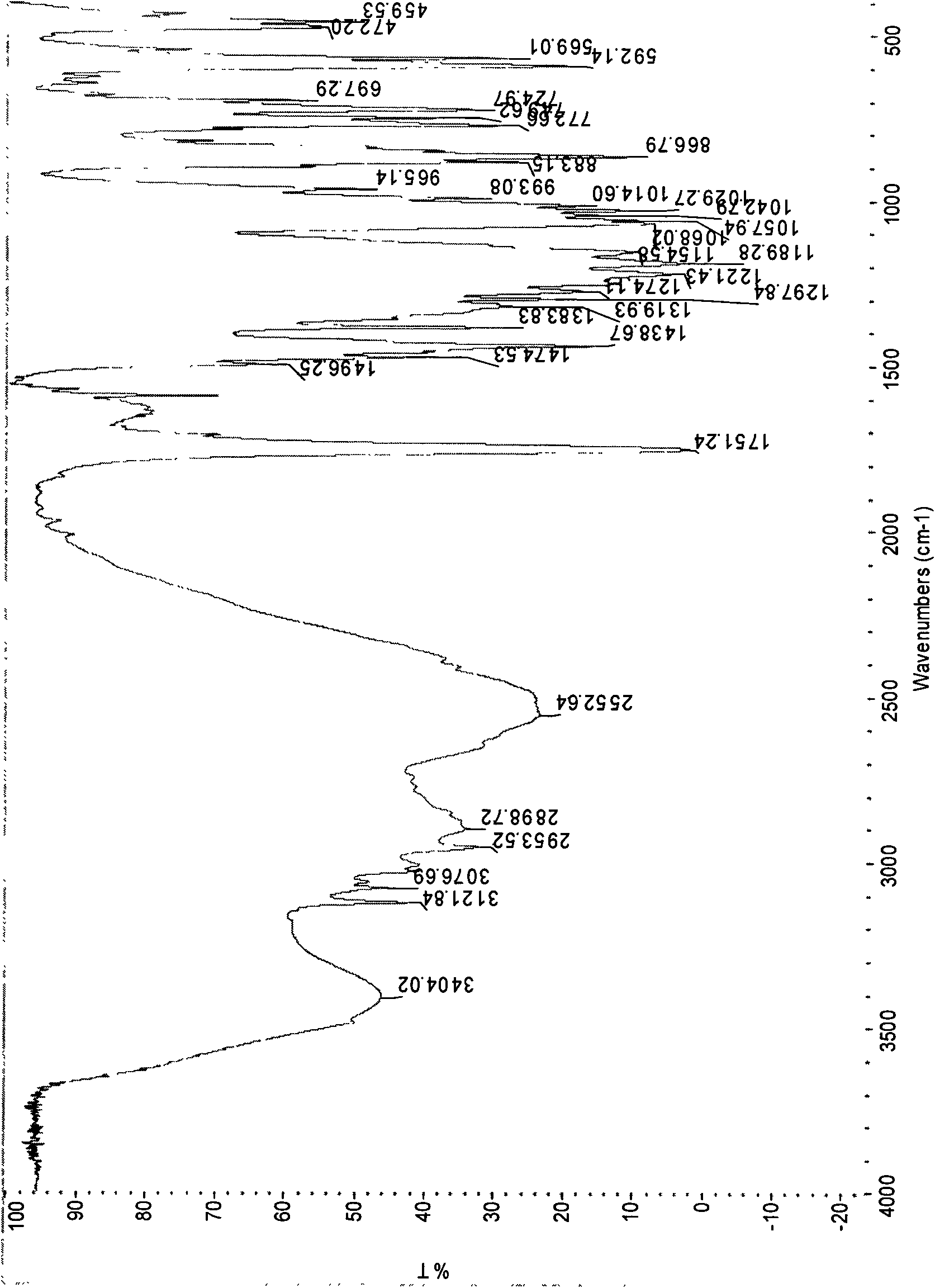
[0047] According to the detection method of United States Pharmacopoeia (USP) to the detection method of clopidogrel bisulfate, the product prepared in this embodiment is fully inspected (but the infrared is the II crystal form), and the specific results are as follows:
[0048] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com