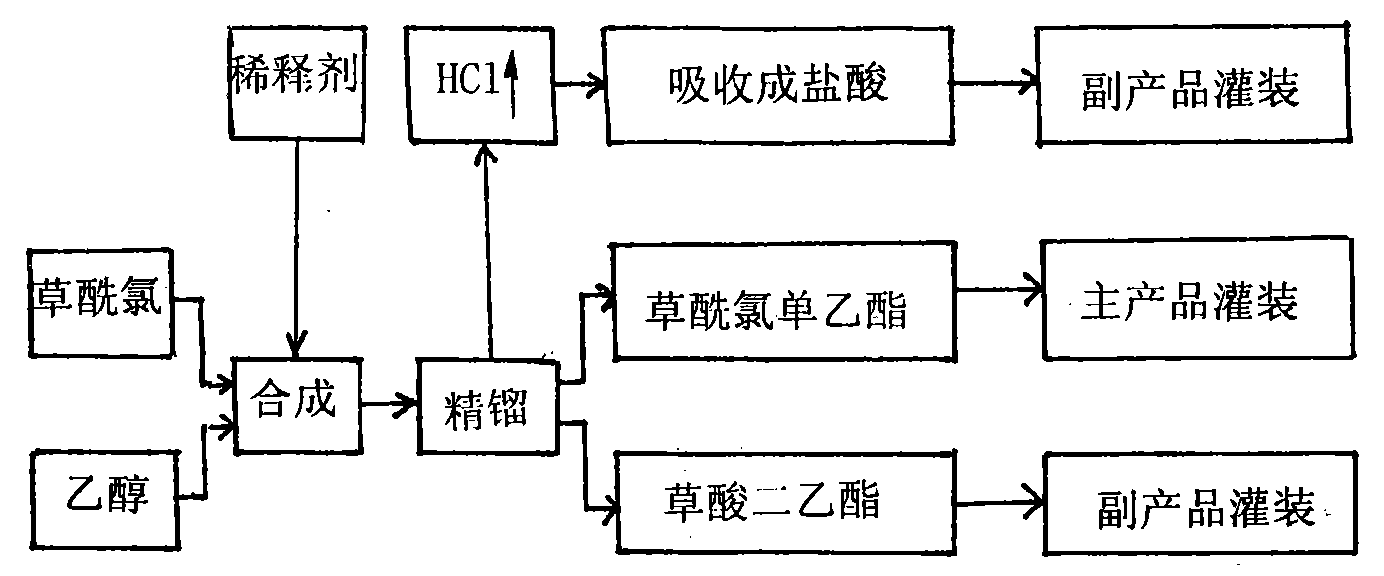
Monoethyl oxalyl chloride and preparation method thereof
A technology for monoethyl oxalyl chloride and a production process, which is applied in the preparation of carboxylic acid halides, organic chemistry, etc., can solve the problems of difficult control of the production process, low product yield, and many by-products, and achieve good product quality and process The effect of simple route and less equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, a kind of monoethyl oxalyl chloride and its production process, take oxalyl chloride and absolute ethanol as raw materials, put diethyl oxalate as a diluent into a reaction kettle, and make it synthesized at a temperature of 24°C-28°C Reaction, the feed ratio of oxalyl chloride and dehydrated alcohol is 2.18, diethyl oxalate is 3.6 times of dehydrated alcohol, and reaction generates product monoethyl oxalyl chloride yield 85%, product purity 99.2% and by-product dioxalate Ethyl ester and hydrochloric acid, the reaction is stable and easy to control.
Embodiment 2
[0021] Embodiment 2, a kind of monoethyl oxalyl chloride and its production process, the raw materials, diluent and by-products are the same as those in Example 1, the synthesis reaction temperature is 20°C-25°C, the molecular ratio of feed is 1.70, and diethyl oxalate is the diluent It is 4.8 times that of absolute ethanol, and the yield of monoethyl oxalyl chloride produced by the reaction is 85.1%, the purity is 99%, and the reaction is stable.
Embodiment 3
[0022] Embodiment 3, a kind of monoethyl oxalyl chloride and its production process, the raw materials, diluent and by-products are the same as in Example 1, the difference is that the reaction temperature is 15°C-19°C, the molecular feed ratio is 2.15, and the diluent is 3.9 times, The yield of the obtained product monoethyl oxalyl chloride is 87%, the purity is 99%, the reaction is stable and easy to control, and the effect is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com