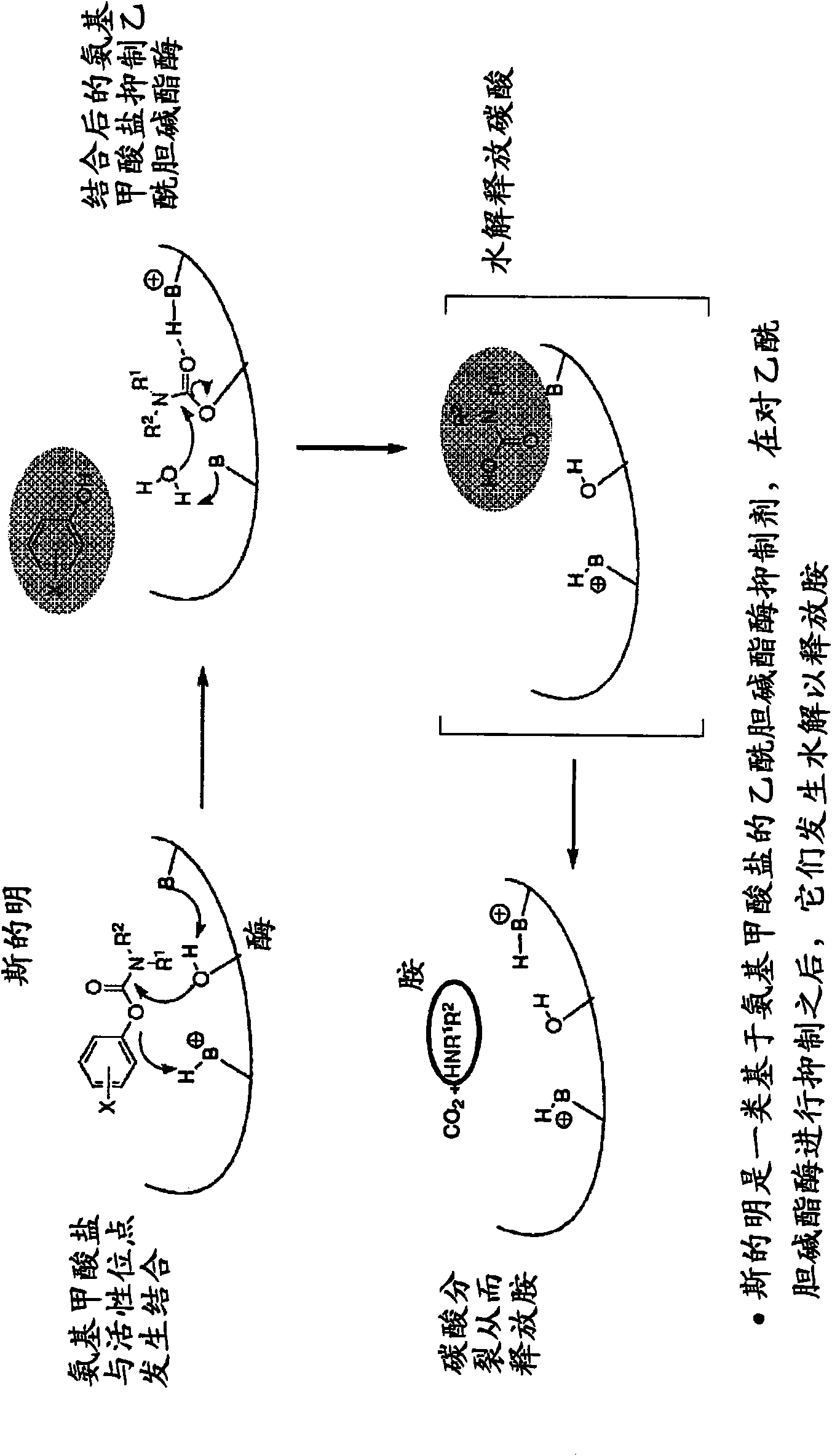
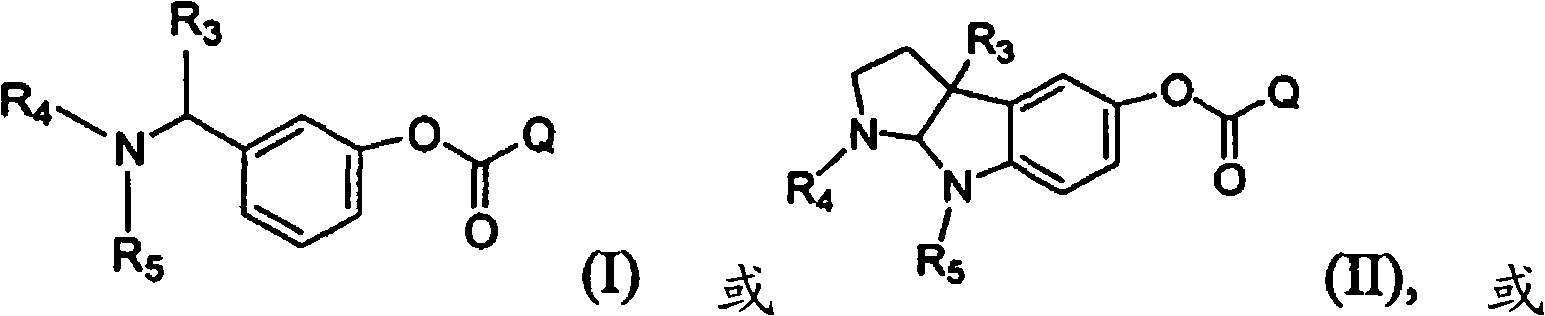
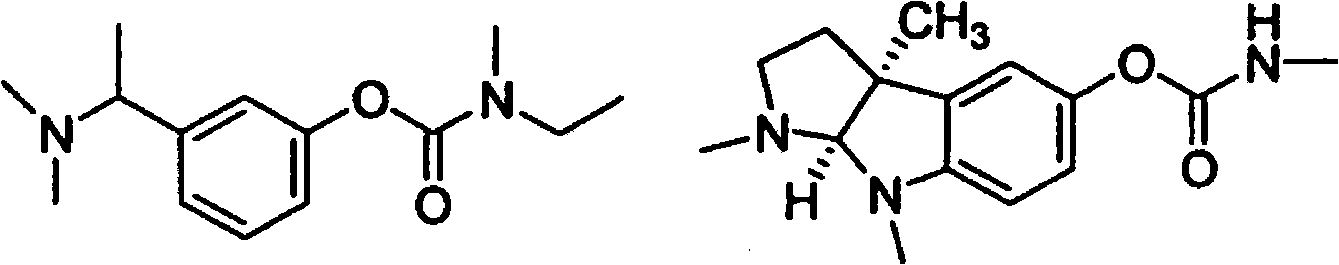
Compounds that inhibit cholinesterase
A technology of acetylcholinesterase and compounds, applied in the direction of drug combination, organic chemistry, ester active ingredients, etc., can solve the problem that drugs cannot be targeted to specific cells or tissues, drug delivery cannot be delivered, and the interruption of single cell signal molecules cannot be effective Treatment of diseases or illnesses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0546] Embodiment 1: the synthesis of compound
[0547] Compounds described in the present invention are subjected to R by methods known to those skilled in the art a - Prepared by the coupling reaction of phenol and Q-H. For example:
[0548]
[0549] where R a represents a suitable phenyl substituent of a Stigmine such as Rivastigmine or Physostigmine, and Q represents a pharmacologically active agent containing an amine. For example,
[0550]
[0551] Exemplary compounds are shown in Table A.
[0552] Table A
[0553] starting material
[0554] fluoxetine hydrochloride
[0555] Sertraline Maleate
[0556] Betahistine dihydrochloride
[0557] Gabapentin
[0558] fluvoxamine maleate
Embodiment 2
[0559] Embodiment 2: the preparation of hydrochloride
Embodiment 2A
[0560] Example 2A: Compounds described in the present invention were dissolved in chloroform (3 ml of chloroform per mmol of compound was used). Thereto was added dropwise a 1M diethyl ether solution of hydrochloric acid (1.5-2 molar equivalents) at 0°C. After the step of adding hydrochloric acid was completed, the mixture was warmed to room temperature. The solvent was removed by evaporation and the residue was dried under vacuum to obtain the hydrochloride salt of the compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com