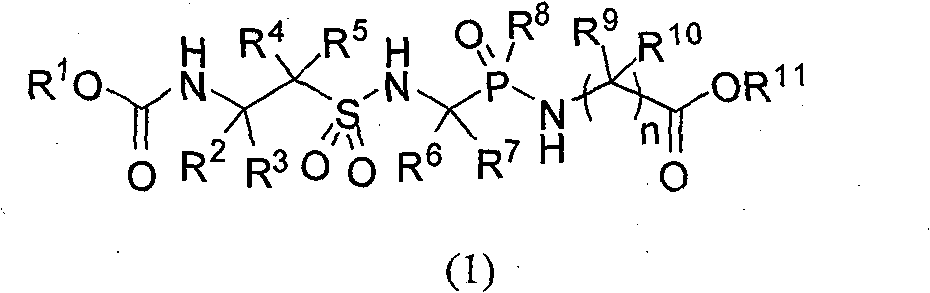
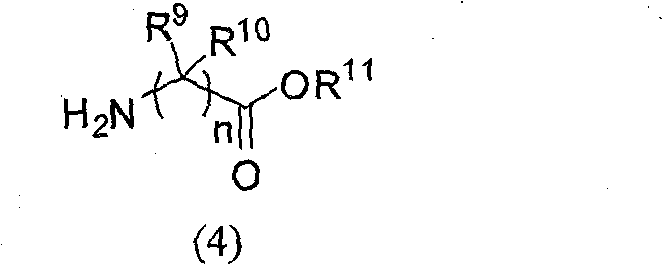
Sulfuryl hypo-phosphono hybridation peptide and preparation method thereof
A technology of sulfonylphosphinate and hybrid peptide, which is applied in the field of sulfonylphosphinate hybrid peptide and its preparation, and can solve the problem that there is no sulfonylphosphinate hybrid peptide yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of N-[N-[N-Benzyloxycarbonylaminoethanesulfonyl]amino-(4-methylphenyl)-benzylphosphinoyl]glycine ethyl ester (1a)
[0048]
[0049]Dissolve 0.49g (1.9mmol) of Cbz-protected taurine in 10mL of anhydrous acetonitrile (slightly heat to promote dissolution), and then add 0.24g (2.0mmol) of p-tolualdehyde and 0.358g (2.0mmol) of benzene Phosphine dichloride, stirred at 45°C for 12h. After the reaction, add 0.824g (8mol) ethyl glycine, stir for 10min and add 1.4mL (0.808g, 8mmol) Et 3 N, after continuing to react for 24h, spin dry acetonitrile. 50 mL of ethyl acetate was added, and the organic phase was washed with 50 mL of saturated NaHCO 3 Wash three times with aqueous solution, wash twice with saturated saline, wash three times with 0.1mol / L citric acid aqueous solution, and wash twice with saturated NaCl aqueous solution. Dry over anhydrous sodium sulfate and spin dry to obtain a yellow viscous oil, add a small amount of ethyl acetate to crystallize in t...
Embodiment 2
[0051] Preparation of N-[N-[N-Benzyloxycarbonylaminoethanesulfonyl]amino-(4-methylphenyl)-benzylphosphinoyl]glycine ethyl ester (1a)
[0052] According to the method described in Example 1, when adding 0.619g (2.4mmol) Cbz protected taurine amide, then adding 0.324g (2.7mmol) p-tolualdehyde and 0.483g (2.7mmol) phenyl dichloro Phosphine. When 1.116 g (8 mol) of glycine ethyl ester hydrochloride was added after the first step of reaction for ammonolysis, the yield was 30%.
Embodiment 3
[0054] Preparation of N-[N-[N-Benzyloxycarbonylaminoethanesulfonyl]amino-(4-methylphenyl)-benzylphosphinoyl]glycine ethyl ester (1a)
[0055] According to the method described in Example 2, when 0.824 g (8 mol) ethyl glycine was added for ammonolysis, the yield was 43%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com