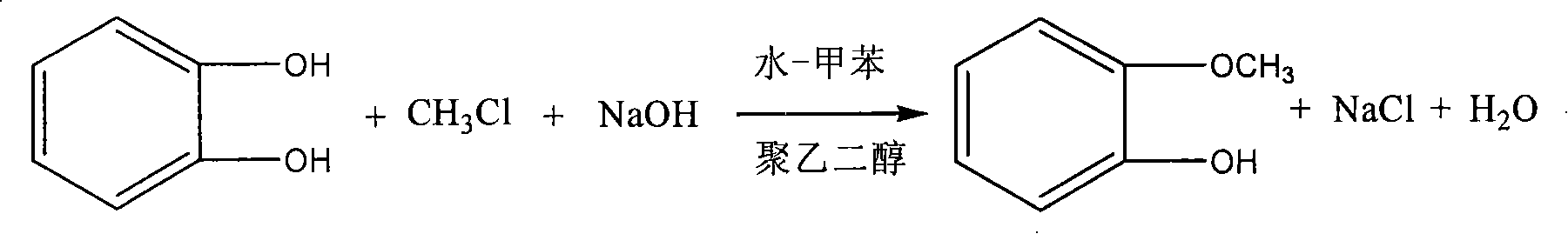
Method for synthesizing guaiacol
A technology of guaiacol and catechol, applied in organic chemistry, ether preparation and other directions, to achieve the effects of high yield, short process flow and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1) After adding 0.2mol catechol, polyethylene glycol-400, 0.18mol sodium hydroxide, 20ml water and 60ml toluene, which account for 1% of the catechol mass, into the reactor, fill it with 0.20mol methyl chloride gas, Stir, the temperature rises to 100°C within 30 minutes and react for 1 hour;
[0021] 2) Add 1:1 hydrochloric acid to the reaction solution in step 1) to adjust the pH=5 to 6, let stand for stratification, weigh the toluene phase, and obtain the yield of guaiacol by gas chromatography analysis is 70.00%, selectivity It was 87.64%, and the conversion rate of catechol was 79.83%.
Embodiment 2
[0023] 1) After adding 0.2mol catechol, polyethylene glycol-6000, 0.22mol sodium hydroxide, 20ml water and 60ml toluene, which account for 4% of catechol mass, into the reactor, fill it with 0.28mol methyl chloride gas, Stirring, the temperature rose to 140°C within 30 minutes and reacted for 4 hours;
[0024] 2) Add 1:1 hydrochloric acid to the reaction solution in step 1) to adjust the pH=5 to 6, let stand for stratification, weigh the toluene phase, and obtain the yield of guaiacol by gas chromatography analysis is 87.24%, selectivity It is 87.24%, and the conversion rate of catechol is 100%.
Embodiment 3
[0026] 1) After adding 0.2mol catechol, polyethylene glycol-6000, 0.22mol sodium hydroxide, 20ml water and 60ml toluene, which account for 5% of catechol mass, into the reactor, fill it with 0.28mol methyl chloride gas, Stirring, the temperature rose to 140°C within 30 minutes and reacted for 4 hours;
[0027] 2) Add 1:1 hydrochloric acid to the reaction solution in step 1) to adjust the pH=5 to 6, let stand for stratification, weigh the toluene phase, and obtain the yield of guaiacol by gas chromatography analysis is 79.81%, selectivity is 85.52%, and the conversion rate of catechol is 93.32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com