Method of replenishing indium ions in indium electroplating compositions
A composition and technology of indium ions, applied in the field of supplementing indium ions, can solve problems such as inability to accurately know the concentration of additives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0064] Embodiment 1 (comparative example)
[0065] Prepare the following aqueous indium compositions:
[0066] Table 1
[0067] components
content
Indium ion ( 3+ ) (from indium sulfate)
60g / L
30g / L
Imidazole-epichlorohydrin copolymer 1
100g / L
water
to the desired volume
pH
1
[0068] 1. Lugalvan TM IZE, obtained from BASF (IZE contains 48-50 wt% copolymer)
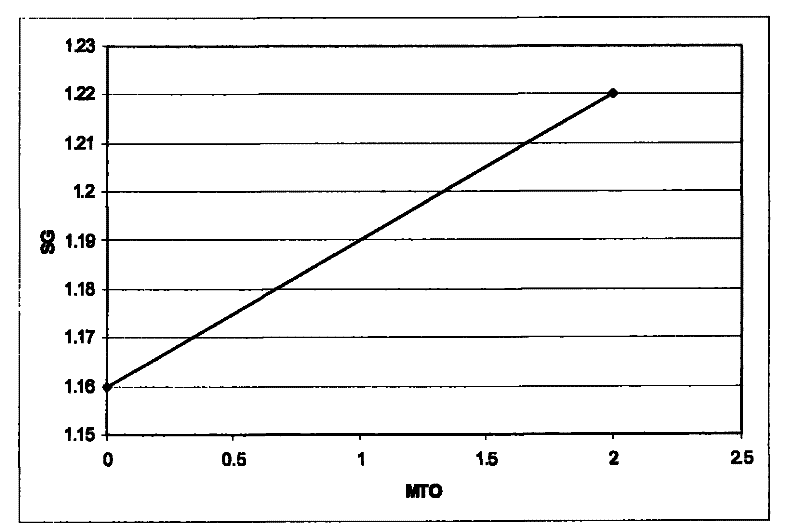
[0069] The indium composition was used to deposit an indium layer on a copper plate. The indium electroplating composition was maintained at a pH of 1 and a temperature of 60°C. The pH was adjusted with KOH. The initial S.G. was measured to be 1.16. The specific gravity is measured with a conventional gas density meter. The composition was continuously stirred during indium metal plating. The cathode current density is maintained at 10A / dm 2 , and the indium deposition rate was 1 μm in 20 seconds. A copper pl...
Embodiment II
[0072] The following aqueous indium electroplating compositions were prepared:
[0073] Table 2
[0074] components
content
Indium ion ( 3+ ) (from indium sulfate)
60g / L
30g / L
Imidazole-epichlorohydrin copolymer 2
100g / L
water
to the desired volume
[0075] pH
1
[0076] 2. Lugalvan TM IZE, obtained from BASF (IZE contains 48-50 wt% copolymer)
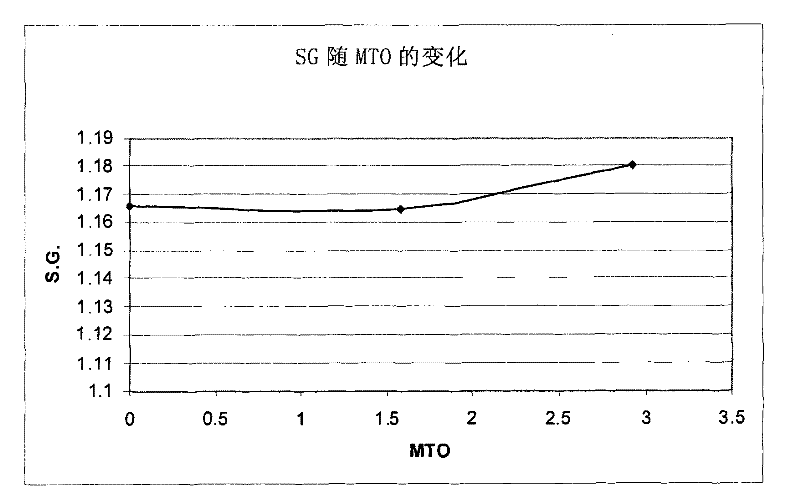
[0077] The indium composition was used to deposit an indium layer on a copper plate. The indium electroplating composition was maintained at a pH of 1 and a temperature of 60°C. The initial S.G. was measured to be 1.165. The composition was continuously stirred during indium metal plating. The cathode current density is maintained at 10A / dm 2 , and the indium deposition rate was 1 μm in 20 seconds. A copper plate served as the cathode, and the anode was a Metakem shielded insoluble anode of titanium and mixed oxides. ...
Embodiment III
[0080] The following aqueous indium electroplating compositions were prepared:
[0081] table 3
[0082] components
content
Indium ion ( 3+ ) (from indium sulfate)
30g / L
30g / L
Imidazole-epichlorohydrin copolymer 3
100g / L
water
to the desired volume
pH
1
[0083] 3. Lugalvan TM IZE, obtained from BASF (IZE contains 48-50 wt% copolymer)
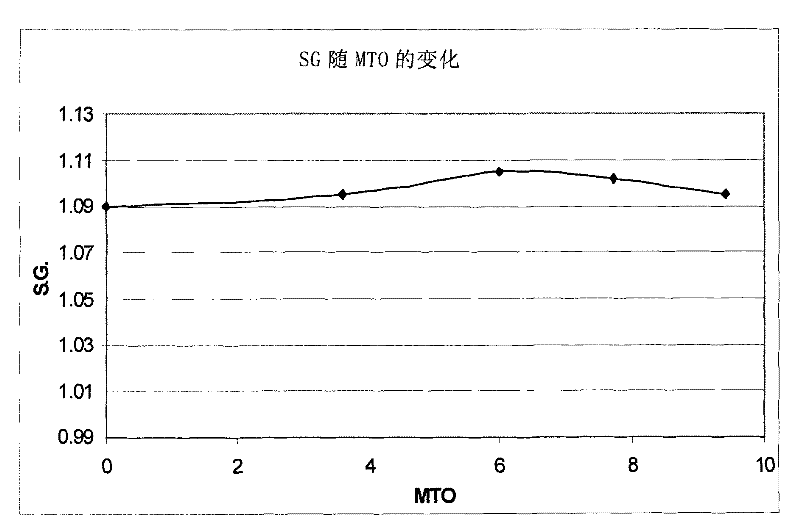
[0084] The indium composition was used to deposit an indium layer on a copper plate. The indium electroplating composition was maintained at a pH of 1 and a temperature of 60°C. The initial S.G. was measured to be 1.09. The composition was continuously stirred during indium metal plating. The cathode current density is maintained at 2A / dm 2 , and the indium deposition rate is 0.6 μm in 1 minute. A copper plate served as the cathode, and the anode was a Metakem shielded insoluble anode of titanium and mixed oxides. During deposition of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com